19.4: Acidic and Basic Character of Carboxylic Acids
- Page ID
- 32523
Objectives
After completing this section, you should be able to
- identify the form that carboxylic acids take within living cells.
- use the Henderson‑Hasselbalch equation to calculate the percentages of dissociated and undissociated acids [A−] and [HA] in a solution, given the pKa value of the acid and the pH of the solution.
- explain why cellular carboxylic acids are always referred to by the name of their anion.
Make certain that you can define, and use in context, the key terms below.
- Henderson‑Hasselbalch equation
- physiological pH
Organic molecules in buffered solution: The Henderson-Hasselbalch equation
The environment inside a living cell, where most biochemical reactions take place, is an aqueous buffer with pH ~ 7 (or sometimes referred to as the physiological pH). Recall from your General Chemistry course that a buffer is a solution of a weak acid and its conjugate base. The key equation for working with buffers is the Henderson-Hasselbalch equation:
The Henderson-Hasselbalch equation:

The equation tells us that if our buffer is an equimolar solution of a weak acid and its conjugate base, the pH of the buffer will equal the pKa of the acid (because the log of 1 is equal to zero). If there is more of the acid form than the base, then of course the pH of the buffer is lower than the pKa of the acid.
The Henderson-Hasselbalch equation is particularly useful when we want to think about the protonation state of different biomolecule functional groups in a pH 7 buffer. When we do this, we are always assuming that the concentration of the biomolecule is small compared to the concentration of the buffer components. (The actual composition of physiological buffer is complex, but it is primarily based on phosphoric and carbonic acids.).
Imagine an aspartic acid residue located on the surface of a protein in a human cell. Being on the surface, the side chain is in full contact with the pH 7 buffer surrounding the protein. In what state is the side chain functional group: the protonated state (a carboxylic acid) or the deprotonated state (a carboxylate ion)?
Using the Henderson-Hasselbalch equation, we fill in our values for the pH of the buffer and a rough pKa approximation of pKa = 5 for the carboxylic acid functional group. Doing the math, we find that the ratio of carboxylate to carboxylic acid is about 100 to 1: the carboxylic acid is almost completely ionized (in the deprotonated state) inside the cell. This result extends to all other carboxylic acid groups you might find on natural biomolecules or drug molecules: in the physiological environment, carboxylic acids are almost completely deprotonated. Indeed, often cellular carboxylic acids are often referred to by the name of their anion. So rather than pyruvic acid, or acetic acid or lactic acid, it is pyruvate, acetate or lactate.
While we are most interested in the state of molecules at pH 7, the Henderson-Hasselbalch equation can of course be used to determine the protonation state of functional groups in solutions buffered to other pH levels. The exercise below provide some practice in this type of calculation.
Exercises
What is the pH of an aqueous buffer solution that is 30 mM in acetic acid and 40 mM in sodium acetate? The pKa of acetic acid is 4.8.
Solution
This is a direct application of the Henderson-Hasselbalch equation.
\[pH = pK _{ a }+\log \left(\dfrac{[\text { concentration of conjugate base }]}{[\text { concentration of weak acid }]}\right) \nonumber \]
The ratio of base to acid is 40/30, or 1.33. Therefore, substituting these values and the \(pK_a\) results in
\[\begin{align*} pH &= 4.8 +\log \left(\dfrac{40}{30}\right) \\[4pt] &= 4.8 +\log 1.33 \\[4pt] &=4.8 + 0.125 \\[4pt] & = 4.9 \end{align*} \]
What is the ratio of acetate ion to neutral acetic acid when a small amount of acetic acid (pKa = 4.8) is dissolved in a buffer of pH 2.8? pH 3.8? pH 4.8? pH 5.8? pH 6.8?
- Answer
-
We use the Henderson-Hasselbalch equation and let the base to acid ratio be \(x\).
For pH = 2.8:
\[2.8 = 4.8 + \log x \nonumber \]
\(x = 0.01 to 1\)
- pH 3.8, the ratio is 0.10 to 1
- pH 4.8, the ratio is 1.0 to 1
- pH 5.8, the ratio is 10 to 1
- pH 6.8, the ratio is 100 to 1
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
The resonance effect described here is undoubtedly the major contributor to the exceptional acidity of carboxylic acids. However, inductive effects also play a role. For example, alcohols have pKa's of 16 or greater but their acidity is increased by electron withdrawing substituents on the alkyl group. The following diagram illustrates this factor for several simple inorganic and organic compounds (row #1), and shows how inductive electron withdrawal may also increase the acidity of carboxylic acids (rows #2 & 3). The acidic hydrogen is colored red in all examples.
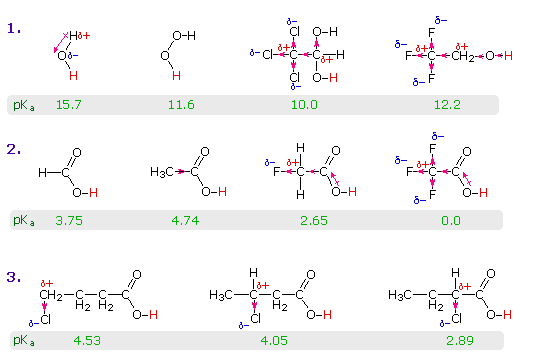
Water is less acidic than hydrogen peroxide because hydrogen is less electronegative than oxygen, and the covalent bond joining these atoms is polarized in the manner shown. Alcohols are slightly less acidic than water, due to the poor electronegativity of carbon, but chloral hydrate, Cl3CCH(OH)2, and 2,2,2,-trifluoroethanol are significantly more acidic than water, due to inductive electron withdrawal by the electronegative halogens (and the second oxygen in chloral hydrate). In the case of carboxylic acids, if the electrophilic character of the carbonyl carbon is decreased the acidity of the carboxylic acid will also decrease. Similarly, an increase in its electrophilicity will increase the acidity of the acid. Acetic acid is ten times weaker an acid than formic acid (first two entries in the second row), confirming the electron donating character of an alkyl group relative to hydrogen, as noted earlier in a discussion of carbocation stability. Electronegative substituents increase acidity by inductive electron withdrawal. As expected, the higher the electronegativity of the substituent the greater the increase in acidity (F > Cl > Br > I), and the closer the substituent is to the carboxyl group the greater is its effect (isomers in the 3rd row). Substituents also influence the acidity of benzoic acid derivatives, but resonance effects compete with inductive effects. The methoxy group is electron donating and the nitro group is electron withdrawing (last three entries in the table of pKa values).
For additional information about substituent effects on the acidity of carboxylic acids Click Here
Contributors
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

