21.10: Malonic Ester Synthesis
- Page ID
- 28437
Malonic ester is a reagent specifically used in a reaction which converts alkyl halides to carboxylic acids called the Malonic Ester Synthesis. Malonic ester synthesis is a synthetic procedure used to convert a compound that has the general structural formula 1 into a carboxylic acid that has the general structural formula 2.

- R1 = alkyl group
- L = leaving group
The group —CH2CO2H in 2 is contributed by a malonic ester, hence the term malonic ester synthesis.
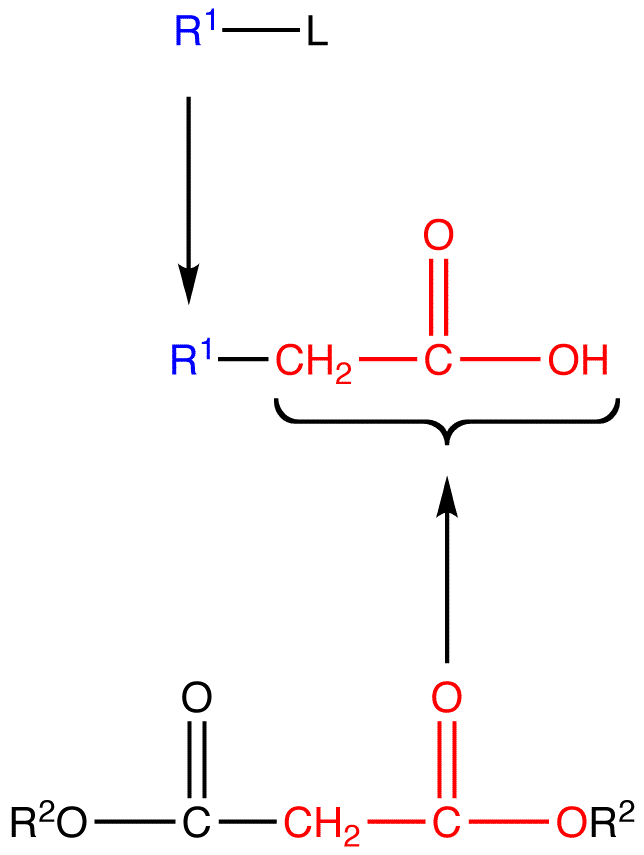
- R2 = alkyl, aryl
Mechanism
Malonic ester synthesis consists of four consecutive reactions that can be carried out in the same pot.
- reaction 1: acid-base reaction
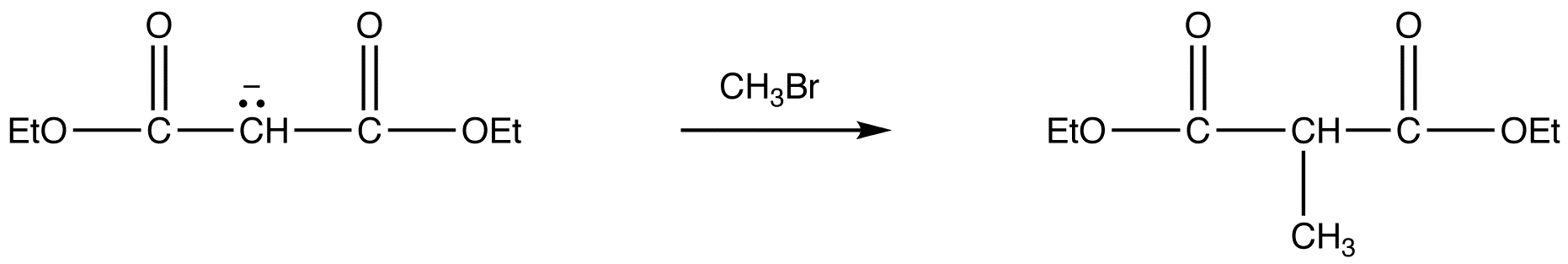
- reaction 2: nucleophilic substitution
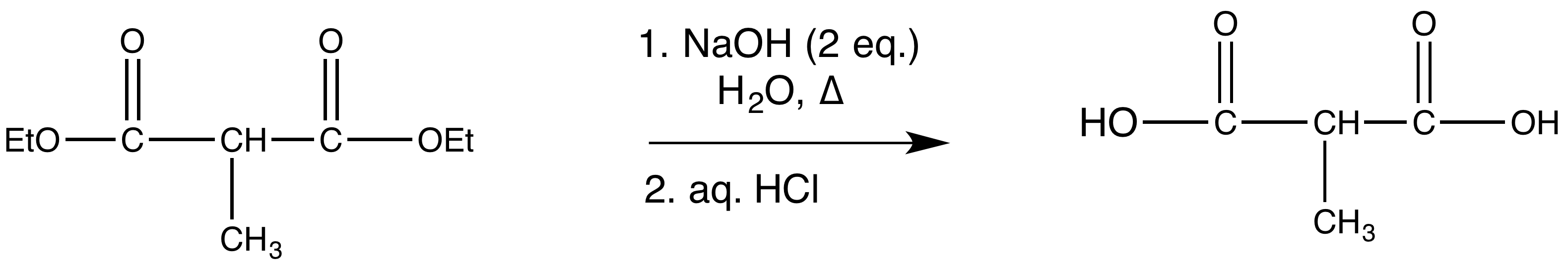
- reaction 3: ester hydrolysis (using saponification)
- reaction 4: decarboxylation
eg:
reaction 1:

reaction 2:
reaction 3:
reaction 4:
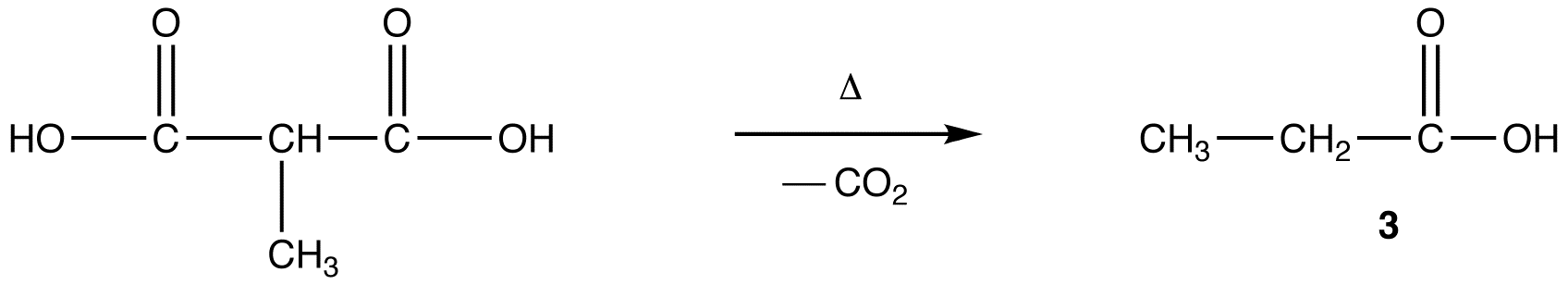
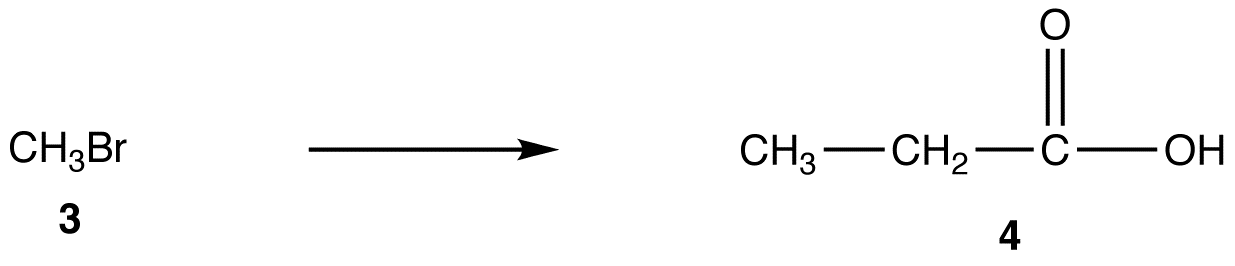
A more direct method to convert 3 into 4 is the reaction of 3 with the enolate ion (5) of ethyl acetate followed by hydrolysis of the resultant ester.
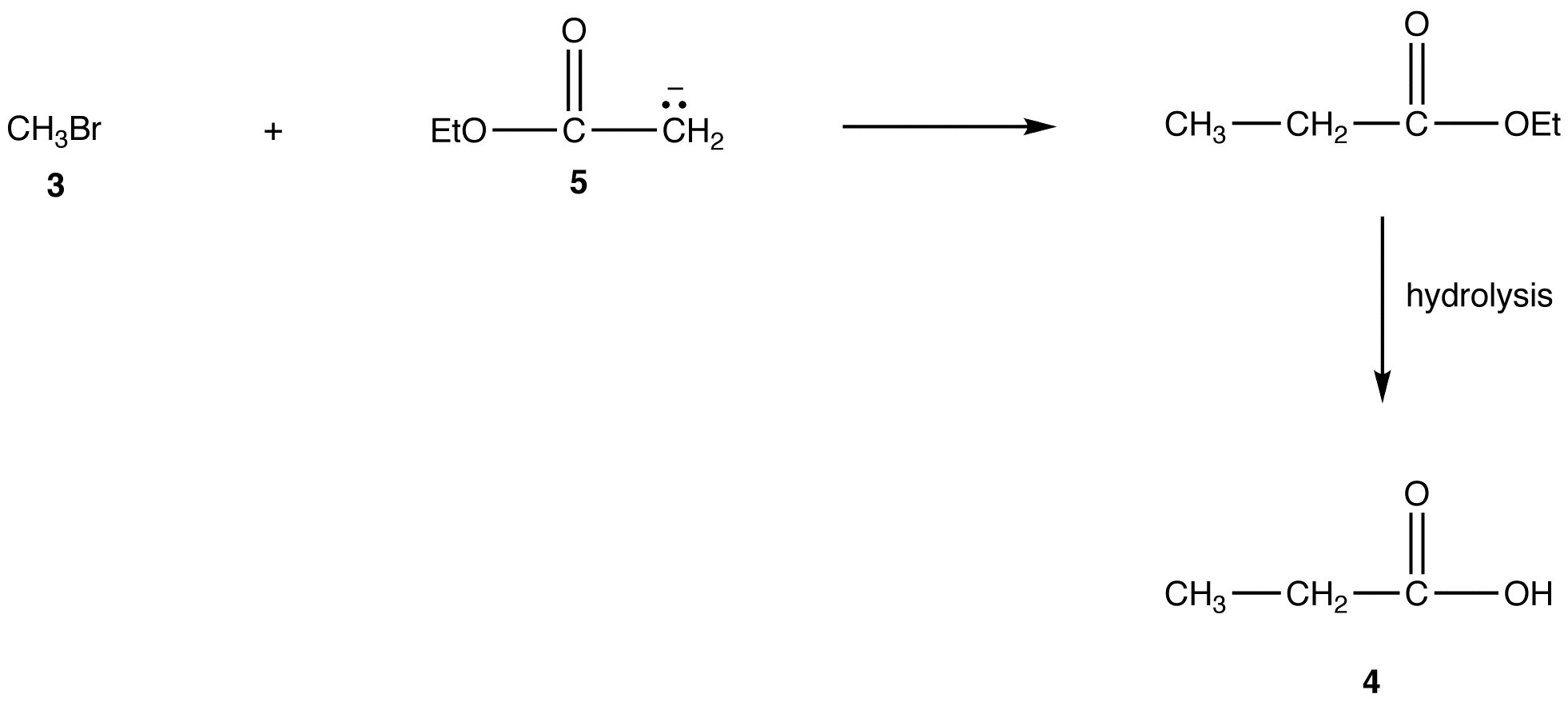
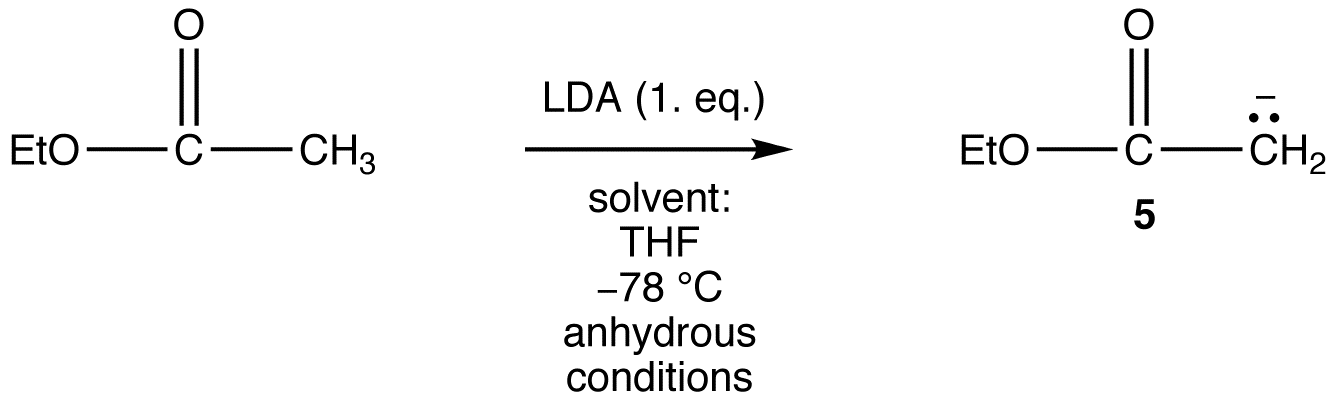
However, the generation of 5 from ethyl acetate quantitatively in high yield is not an easy task because the reaction requires a very strong base, such as LDA, and must be carried out at very low temperature under strictly anhydrous conditions.
Malonic ester synthesis provides a more convenient alternative to convert 3 to 4.
Malonic ester synthesis can be adapted to synthesize compounds that have the general structural formula 6.

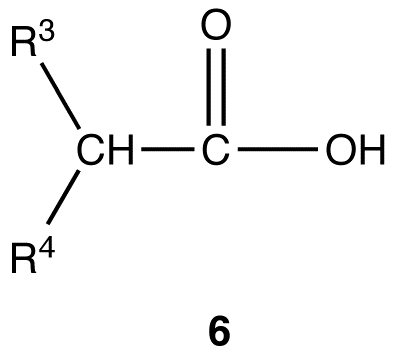
R3, R4 = identical or different alkyl groups
eg:
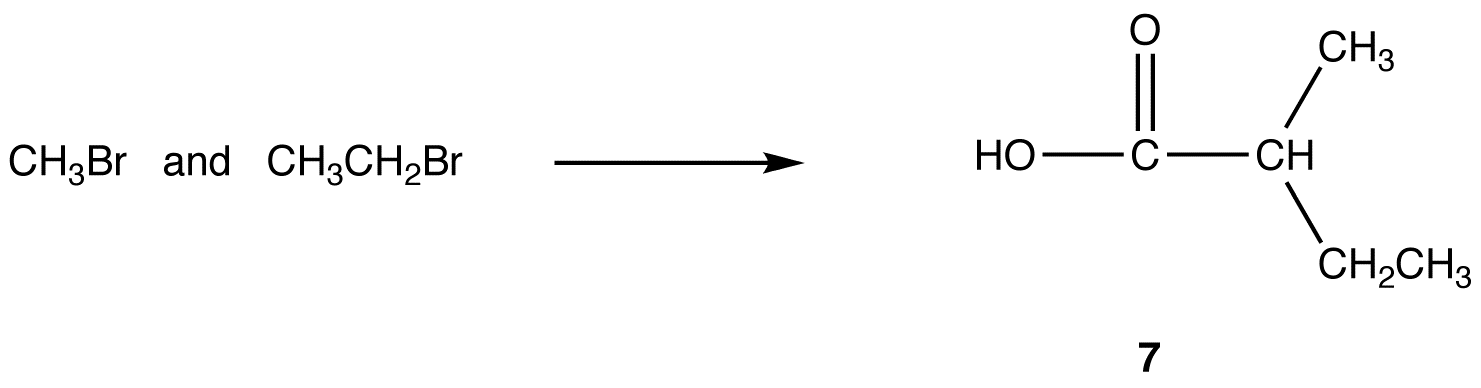
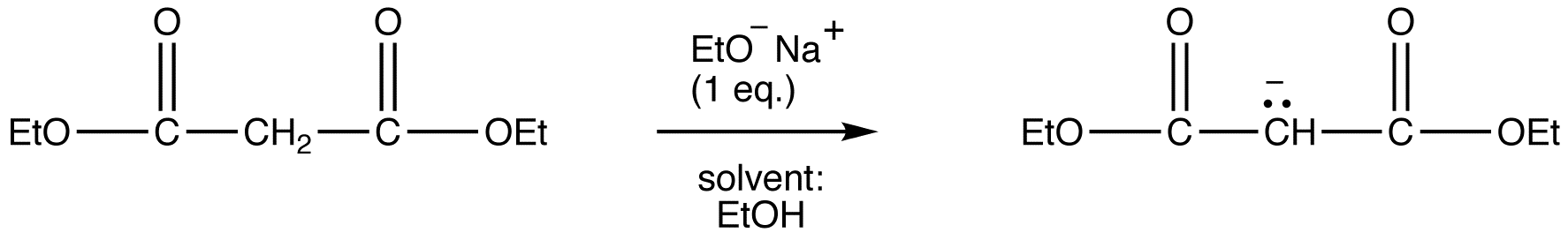
reaction 1:
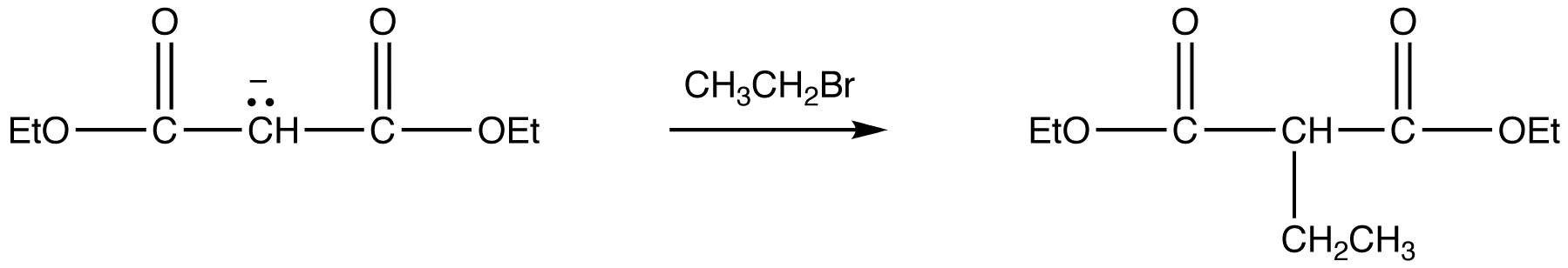
reaction 2:
reaction 1 (repeat):
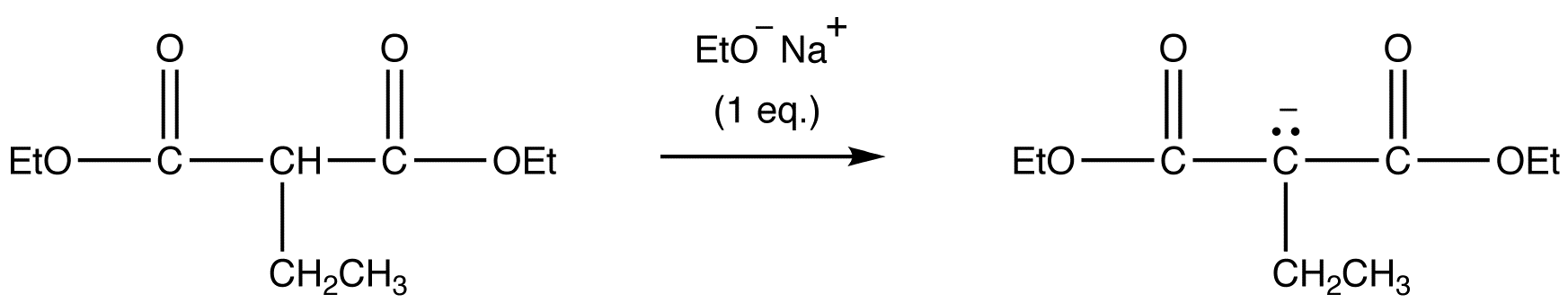
reaction 2 (repeat):
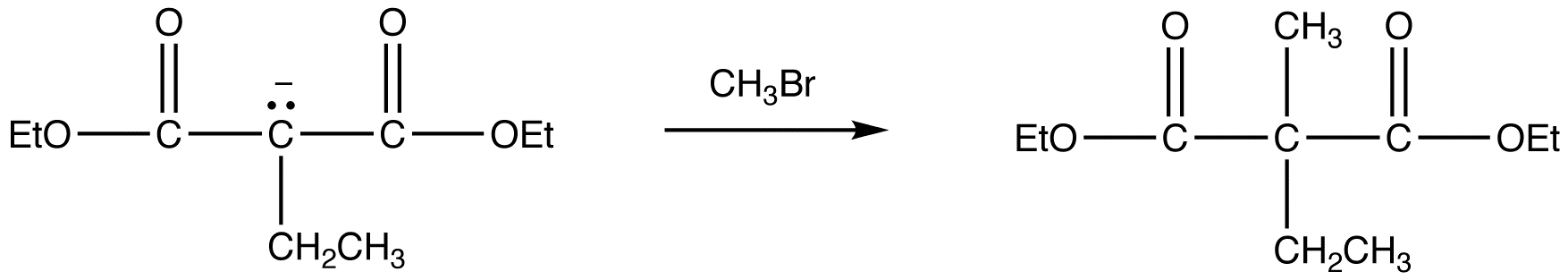
reaction 3:
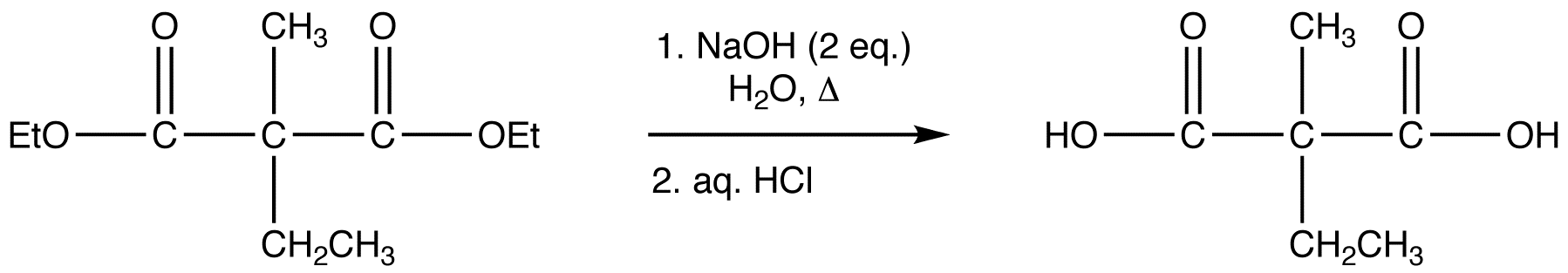
reaction 4:
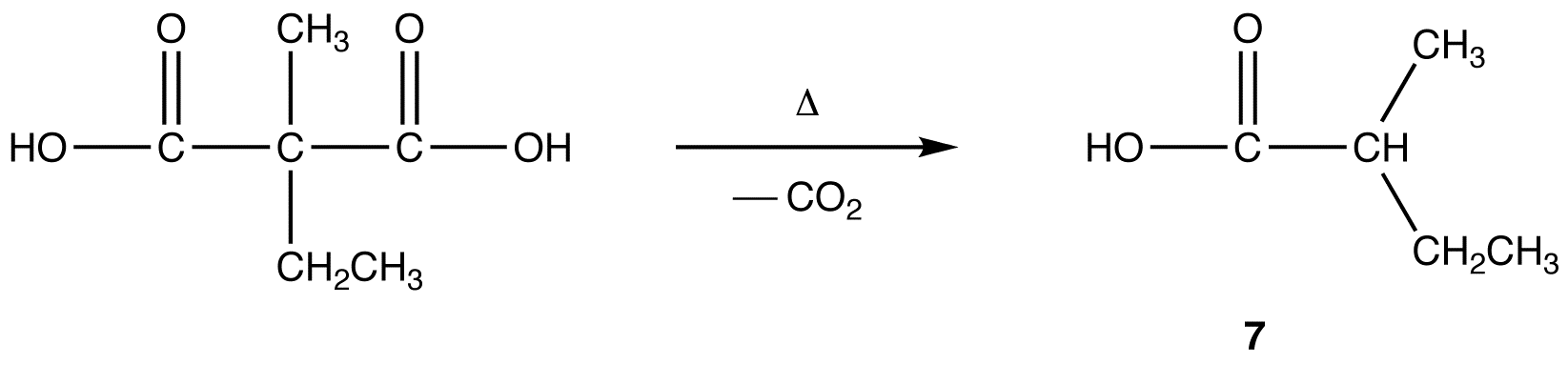
Malonic Ester Synthesis

Due to the fact that Malonic ester’s α hydrogens are adjacent to two carbonyls, they can be deprotonated by sodium ethoxide (NaOEt) to form Sodio Malonic Ester.

Because Sodio Malonic Ester is an enolate, it can then be alkylated with alkyl halides.
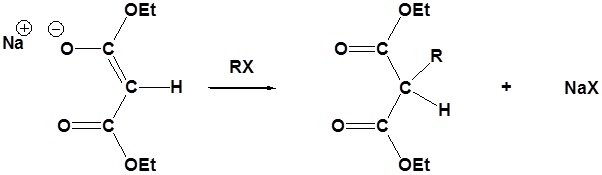
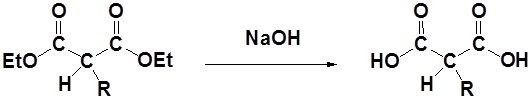
After alkylation the product can be converted to a dicarboxylic acid through saponification and subsequently one of the carboxylic acids can be removed through a decarboxylation step.
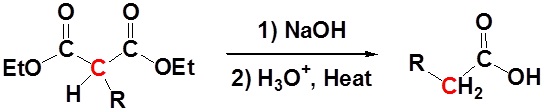
Mechanism
1) Saponification
2) Decarboxylation
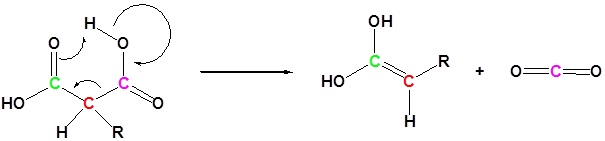
3) Tautomerization

All of the steps together form the Malonic ester synthesis.
\[RX \rightarrow RCH_2CO_2H\]
Example
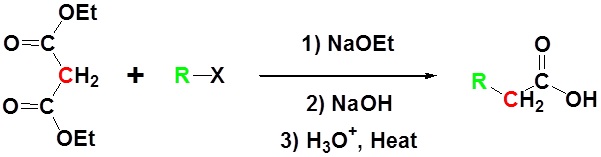
- Bernard E. Hoogenboom , Phillip J. Ihrig , Arne N. Langsjoen , Carol J. Linn and Stephen D. Mulder, The malonic ester synthesis in the undergraduate laboratory, J. Chem. Educ., 1991, 68 (8), p 689
Contributors
Prof. Steven Farmer (Sonoma State University)
- Gamini Gunawardena from the OChemPal site (Utah Valley University)

