27.7: Biosynthesis of Steroids
- Page ID
- 36488
After completing this section, you should be able to
- give an overview of how steroids are synthsized.
- describe changes to molecules through the biosynthesis of steroids.
Steroid biosynthesis is an anabolic metabolic pathway that produces steroids from simple precursors. A unique biosynthetic pathway is followed in animals compared to many other organisms, making the pathway a common target for antibiotics and other anti-infective drugs. In humans and other animals, the biosynthesis of steroids follows the mevalonate pathway that uses acetyl-CoA as building blocks to form dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP). These two compounds are coupled to form geranyl pyrophosphate (GPP) which is then converted to the hydrocarbon polyene squalene. After an oxidation and multiple rearrangements, lanosterol the first steroid in the pathway is formed. Further conversions can yield different steroids.
The key step in the biosynthesis of lanosterol is the regioselective epoxidation of squalene to give (3S)-2,3-oxidosqualene. This reaction is catalyzed by the monooxygenase enzyme squalene epoxidase. In this step, flavin hydroperoxide serves as the direct oxidizing agent. In flavin hydroperoxide, the peroxide group is linked to one of the carbons of the reactive triple-ring system of the coenzyme. A possible mechanism for the formation of flavin peroxide from FADH2 and molecular oxygen is shown below.
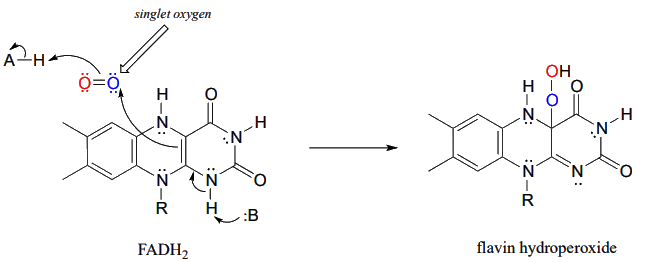
The mechanism for the flavin-hydroperoxide-dependent epoxidation of squalene is initiated by the nucleophilic attack of the pi electrons of a squalene double bond on the electrophilic terminal hydroperoxide oxygen.
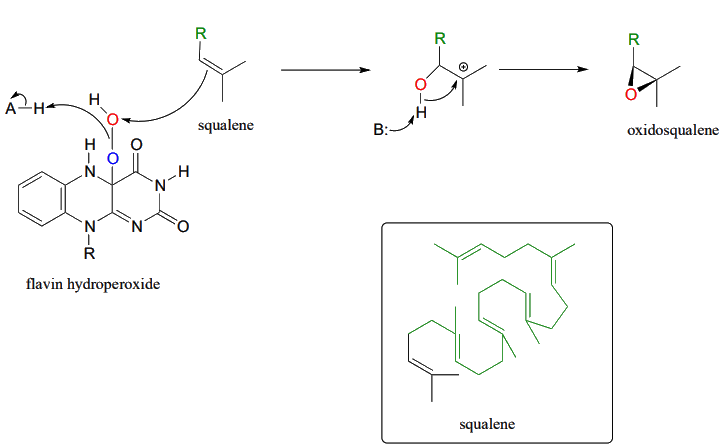 .
.
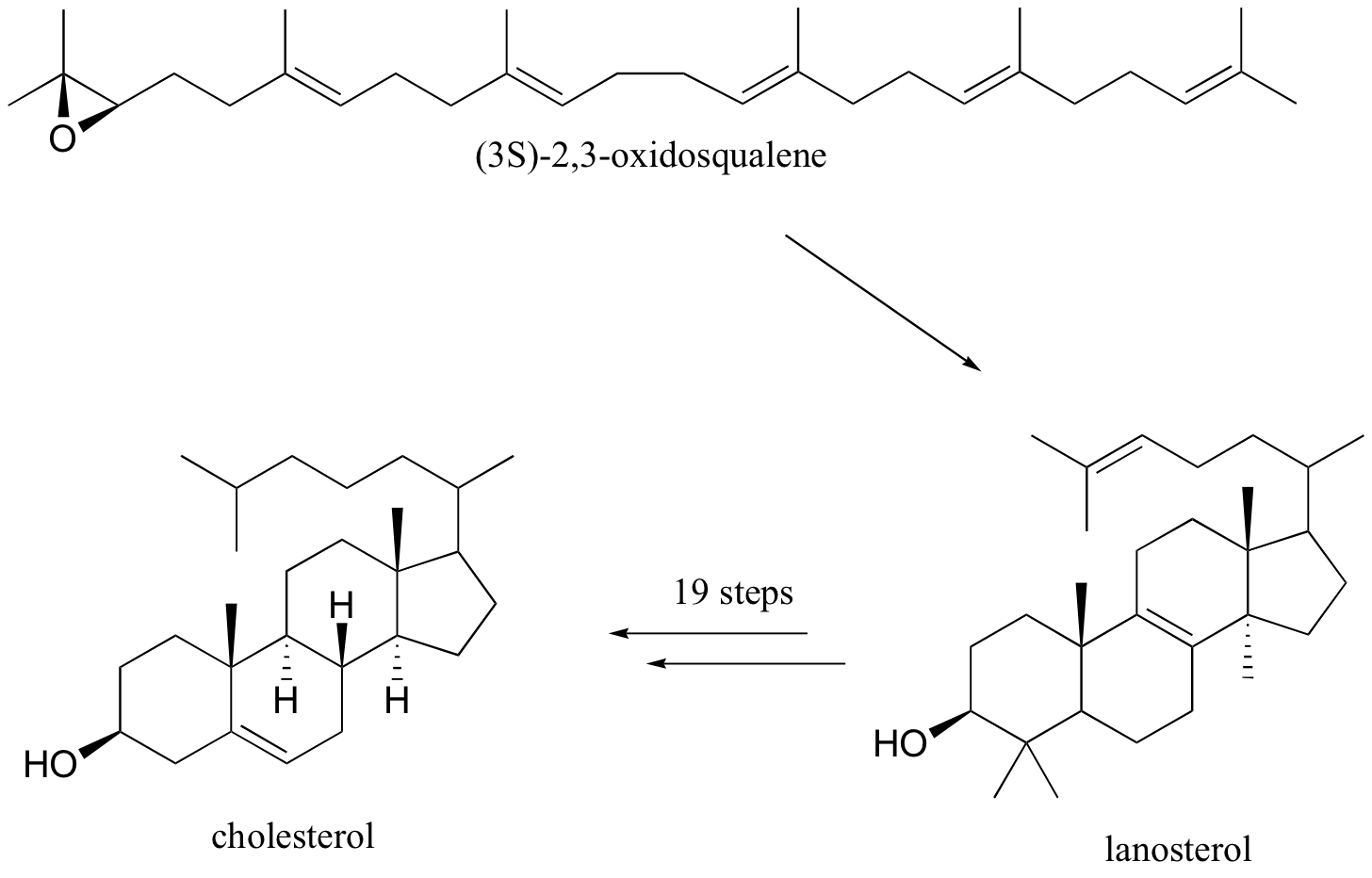
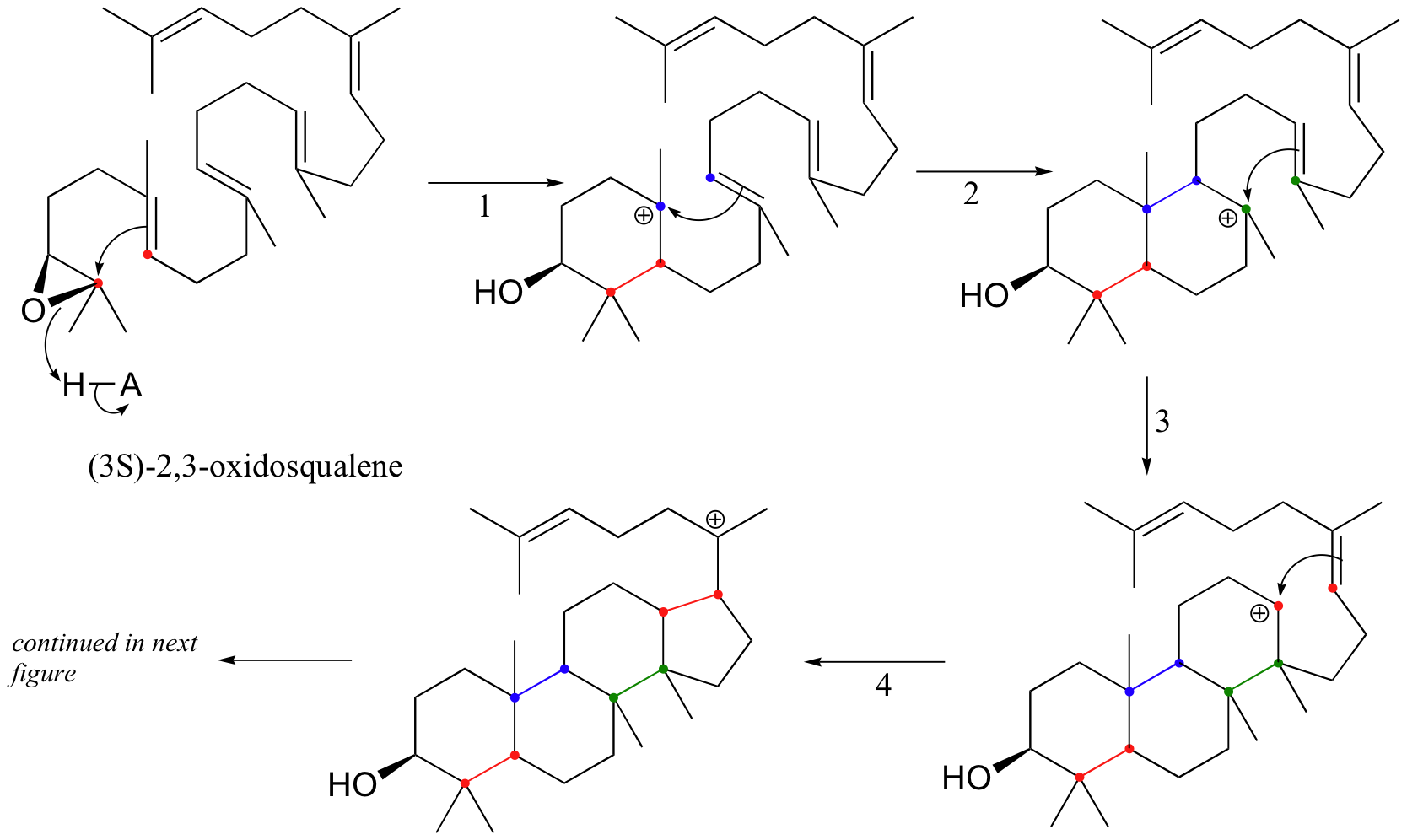
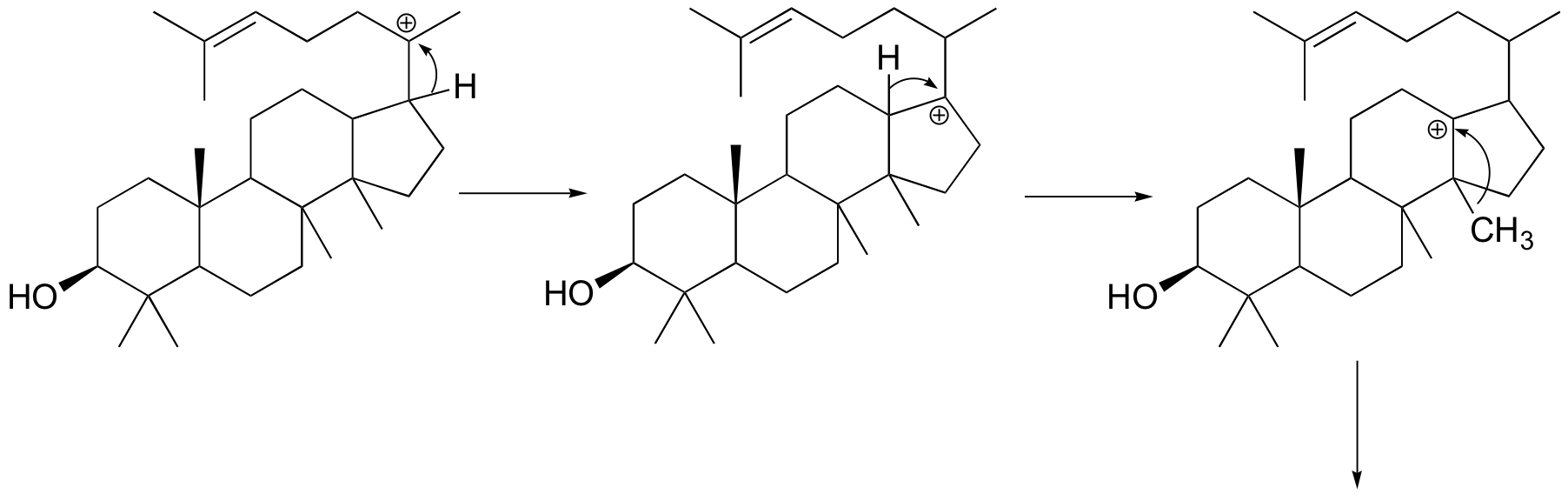
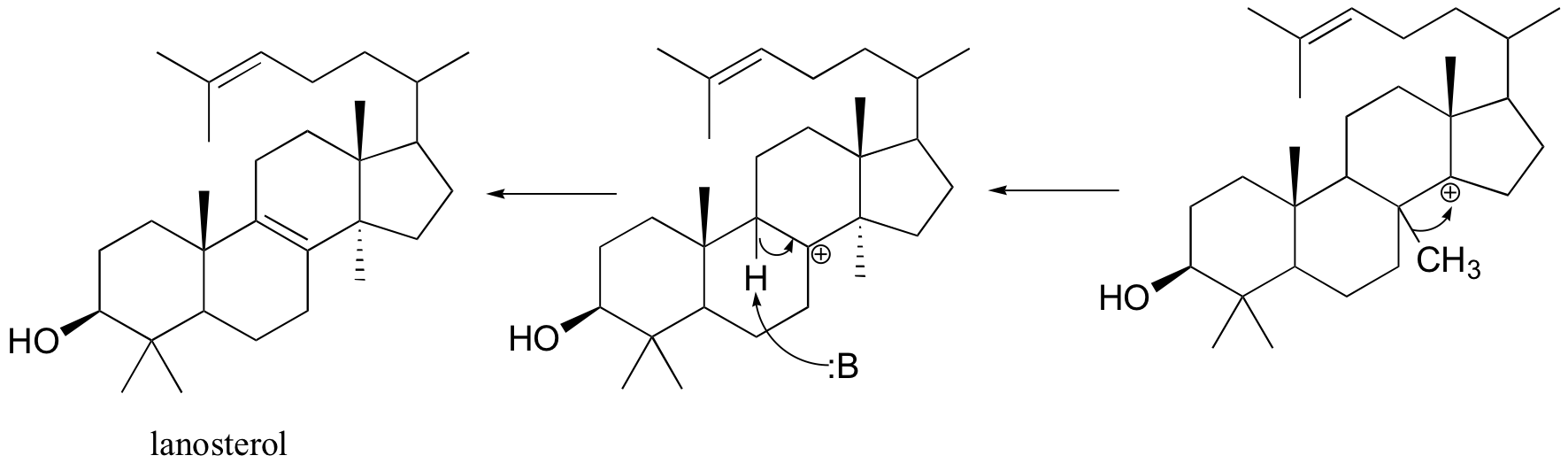
Oxidosqualene goes on to cyclize to lanosterol in a complex and fascinating electrophilic reaction which catalyzed by oxidosqualene-lanosterol cyclase. This reaction has two phases. The first phase, in which the actual cyclization takes place, is a series of electrophilic addition steps. The second phase is a series of hydride and methyl shifts. There is some argument about whether these processes occur in a stepwise fashion (with discreet carbocation intermediates) or in a concerted manner. For the sake of clarity, we will show the reaction proceeding stepwise. After lanosterol is formed the pathway continued to form cholesterol which is the precursor that all other steroids are derived.
The cyclization phase begins with attack by pi electrons on an epoxide electrophile. The epoxide ring opens to form an alcohol after protonation. A relatively stable tertiary carbocation is also formed. Steps 2, 3, and 4 are simply successive attacks by pi electrons on the carbocation generated by the previous attack. The overall result of this electrophilic cascade is the opening of the epoxide ring, and closure of three six-membered and one five-membered ring.
Next comes the rearrangement phase of the reaction in which a series of two 1,2-hydride and two 1,2-methyl shifts to give lanosterol after proton loss from the 9 position.
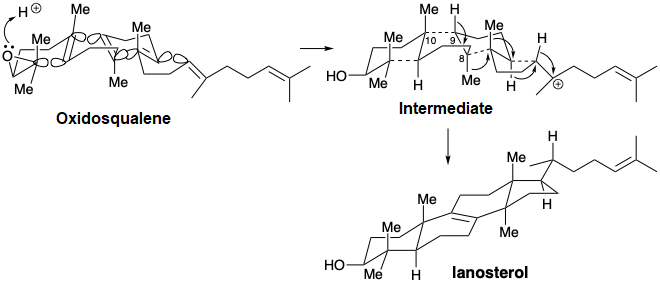
Especially interesting are the stereochemical details of the polyene cyclization and subsequent rearrangement. The polyene cyclization involves stereospecific anti-periplanar addition across three C=C bonds in oxidosqualene (see below). The folded conformation of oxidosqualene, required for cyclization is probably imposed by the enzyme involved since appreciable steric congestion is present in both oxidosqualene and the intermediate. Relief of steric strain provides a large driving force for the rearrangements to lanosterol that involves stereospecific inversion of configuration at each stereocenter during 1,2-hydrogen or methyl shifts. Thus, each 1,2-hydride or 1,2-methyl shift occurs to the backside of the orbital connected to the leaving group in what can be viewed as a series of nucleophilic substitution reactions – where σ-bonding electron pairs serve as the nucleophiles.
What changes must be made to the structure of lanosterol to convert it to cholesterol?
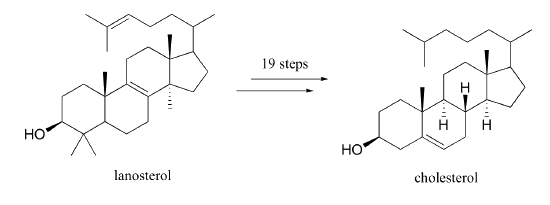
- Answer
-
Three methyl groups and a double bond are removed. Another double bond is moved to the other side of a six-membered ring.

