25.6: Reactions of Monosaccharides
- Page ID
- 36458
After completing this section, you should be able to
-
- write equations to illustrate that the hydroxyl groups of carbohydrates can react to form esters and ethers.
- identify the product formed when a given monosaccharide is reacted with acetic anhydride or with silver oxide and an alkyl halide.
- identify the reagents required to convert a given monosaccharide to its ester or ether.
-
- write an equation to show how a monosaccharide can be converted to a glycoside using an alcohol and an acid catalyst.
- identify the product formed when a given monosaccharide is treated with an alcohol and an acid catalyst.
- write a detailed mechanism for the formation of a glycoside by the reaction of the cyclic form of a monosaccharide with an alcohol and an acid catalyst.
- identify the ester formed by phosphorylation in biologically important compounds.
-
- identify the product formed when a given monosaccharide is reduced with sodium borohydride.
- identify the monosaccharide which should be reduced in order to form a given polyalcohol (alditol).
-
- explain that a sugar with an aldehyde or hemiacetal can be oxidized to the corresponding carboxylic acid (also known as aldonic acid). Note: The sugar is able to reduce an oxidizing agent, and is thus called a reducing sugar. Tests for reducing sugars include the use of Tollens’ reagent, Fehling’s reagent and Benedict’s reagent.
- explain why certain ketoses, such as fructose, behave as reducing sugars even though they do not contain an aldehyde group.
- identify warm HNO3 as the reagent needed to form dicarboxylic acid (an aldaric acid).
-
- describe the chain-lengthening effect of the Kiliani-Fischer synthesis.
- predict the product that would be produced by the Kiliani-Fischer synthesis of a given aldose.
- identify the aldose that would yield a given product following Kiliani-Fischer synthesis.
-
- describe the chain-shortening effect of the Wohl degradation.
- predict the product that would be produced by the Wohl degradation of a given aldose.
- identify the aldose or aldoses that would yield a given product following Wohl degradation.
Make certain that you can define, and use in context, the key terms below.
- aldaric acid
- aldonic acid
- alditol
- aldonic acid
- glycoside
- Kiliani-Fischer synthesis
- neighbouring group effect
- reducing sugar
- Wohl degradation
While several reactions are covered in this section, keep in mind that you have encountered them in previous sections. The active functional groups on monosaccharides are essentially carbonyls and hydroxyls. Although they now are a part of much larger molecules, their chemistry should be familiar.
The formation of esters and ethers is quite straightforward and should not require further clarification.
Note that glycosides are in fact acetals, and that glycoside formation is therefore analogous to acetal formation. To refresh your memory about the chemistry of acetals, quickly review Section 19.10
Monosaccharides contain both alcohol and carbonyl functional groups. This allows monosaccharides to undergo many of the reactions typical for these functional groups. In particular, alcohols can be converted to esters, converted to ethers, converted to acetals, or oxidized. Carbonyls can be reacted with nucleophiles, be reduced to form alcohols, or be oxidized to form carboxylic acids.
Ester and Ether Formation
The hydroxyl groups of monosaccharides are often converted to ether or ester groups to increase solubility in organic solvents. Also, the conversion allows the sugar to be easily purified and crystallized.
The -OH groups on a monosaccharide can be readily converted to esters and ethers. Esterfication can be done with an acid chloride (Section 21.4) or acid anhydride (Section 21.5) in the presence of a base. During these reactions all of the -OH groups are converted to esters. The monosaccharide, D-glucopyranose can be coverted to the pentacetate through reaction with acetic anhydride.
Treatment of carbohydrates with an alkyl halide by a Williamson ether synthesis (Section 18.2) leads to the formation of ethers. The strongly basic conditions typically used for the Williamson ether synthesis can degrade some sugar molecules. Instead, milder bases or silver oxide (Ag2O) are used to provide ethers in high yields. The monosaccharide, D-glucopyranose can be converted to the pentamethyl ether through reaction with iodomethane and silver oxide.
Glycoside Formation
Reacting a hemiacetal with an alcohol and an acid catalyst produces an acetal in which the anomeric hydroxide has been replaced by an ether
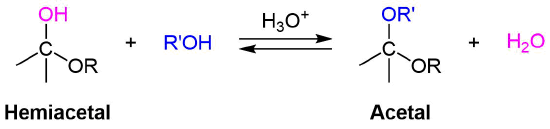
Monosaccharide acetal derivatives, called glycosides, are formed when a hemiacetal reacts with an alcohol in the presence of an acid catalyst. During the reaction the -OH group from the anomeric carbon is replaced by a -OR group from the alcohol. A mixture of alpha and beta products are formed regardless of the conformation of the reactant. In naming of glycosides, the "ose" suffix of the sugar name is replaced by "oside", and the alcohol group name is placed first. This reaction is illustrated below for D-glucopyranose and methanol which forms a mixture of alpha and beta methyl-glucopyranosides. As is generally true for most acetals, glycoside formation involves the loss of an equivalent of water. The acetal product is stable to base and alkaline oxidants such as Tollen's reagent. They are not in equilibrium with the open-chain form and thus do not undergo mutarotation. Since this acid-catalyzed reaction is reversible, glycosides may be hydrolyzed back to their alcohol and sugar components by aqueous acid.
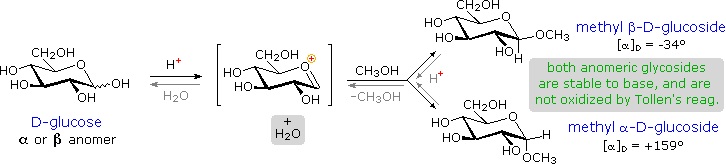
Two examples of naturally occurring glycosides and one example of an amino derivative are displayed below.
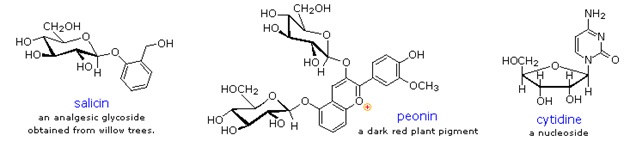
- Salicin, one of the oldest herbal remedies known, was the model for the synthetic analgesic aspirin.
- A large class of hydroxylated, aromatic oxonium cations called anthocyanins provide the red, purple and blue colors of many flowers, fruits and some vegetables. Peonin is one example of this class of natural pigments, which exhibit a pronounced pH color dependence. The oxonium moiety is only stable in acidic environments, and the color changes or disappears when base is added. The complex changes that occur when wine is fermented and stored are in part associated with glycosides of anthocyanins.
- Amino derivatives of ribose, such as cytidine play important roles in biological phosphorylating agents, coenzymes and information transport and storage materials.
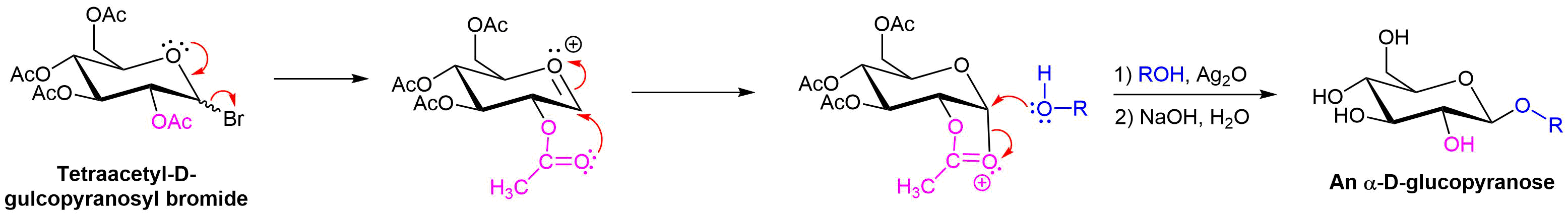
Koenigs–Knorr Reaction
The presence of multiple -OH groups on a sugar molecular makes the synthesis of glycosides particularly difficult. One of the oldest glycosylation reactions, called the Koenigs–Knorr reaction, is effective for preparing the beta-glycosides of glucose. The pathway starts with the reaction of glucose pentaacetate with HBr to form a pyranosyl bromide. Addition of silver oxide allows for the nucleophilic addition of the chosen alcohol. Hydrolysis of the remaning acetal groups forms the beta-glucopyranoside.
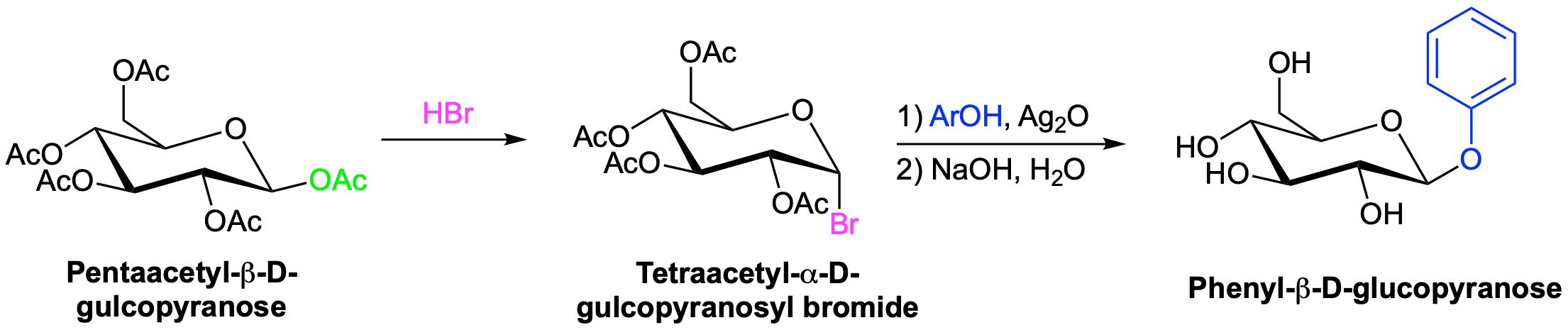
Although the final step of the reaction appears to show the inversion characteristic of an SN2 reaction, the same product forms if either the alpha or beta
Koenigs–Knorr Reaction Mechanism
The mechanism starts with the SN1 like removal of the bromine leaving group which is promoted by the formation of an oxonium ion intermediate. Either anomer of the glucopyranosyl bromide will produce the same oxonium intermediate. Lone pair electrons from the carbonyl oxygen of an adjacent acetate group adds to the oxonium ion in an internal ring-forming reaction. Because the adjacent acetate was on the bottom of the glucose ring the newly form C-O bond is also on the bottom. During this step a new oxonium ion is formed. The alcohol then displaces the oxonium ion as a leaving groups during an SN2 reaction. The inversion of configuration of the SN2 reaction produces a beta-glycoside. Participation of an adjacent acetate group in the mechanism of the Koenigs–Knorr reaction is called a neighboring-group effect.
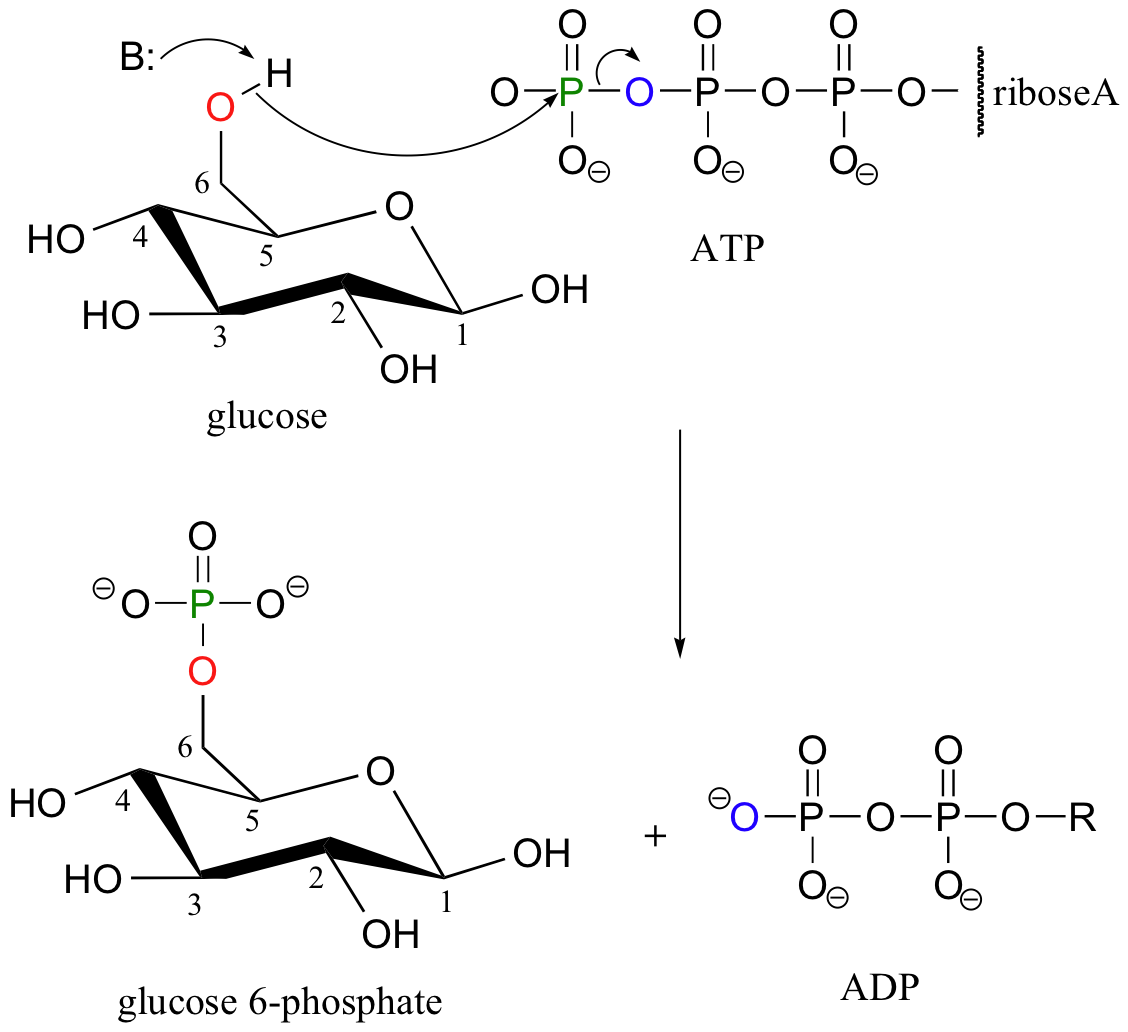
Biological Ester Formation: Phosphorylation
Recall that almost all biomolecules are charged species, which 1) keeps them water soluble, and 2) prevents them from diffusing across lipid bilayer membranes. Although many biomolecules are ionized by virtue of negatively charged carboxylate and positively charged amino groups, the most common ionic group in biologically important organic compounds is phosphate - thus the phosphorylation of alcohol groups is a critical metabolic step. In alcohol phosphorylations, ATP is almost always the phosphate donor, and the mechanism is very consistent: the alcohol oxygen acts as a nucleophile, attacking the gamma-phosphorus of ATP and expelling ADP.
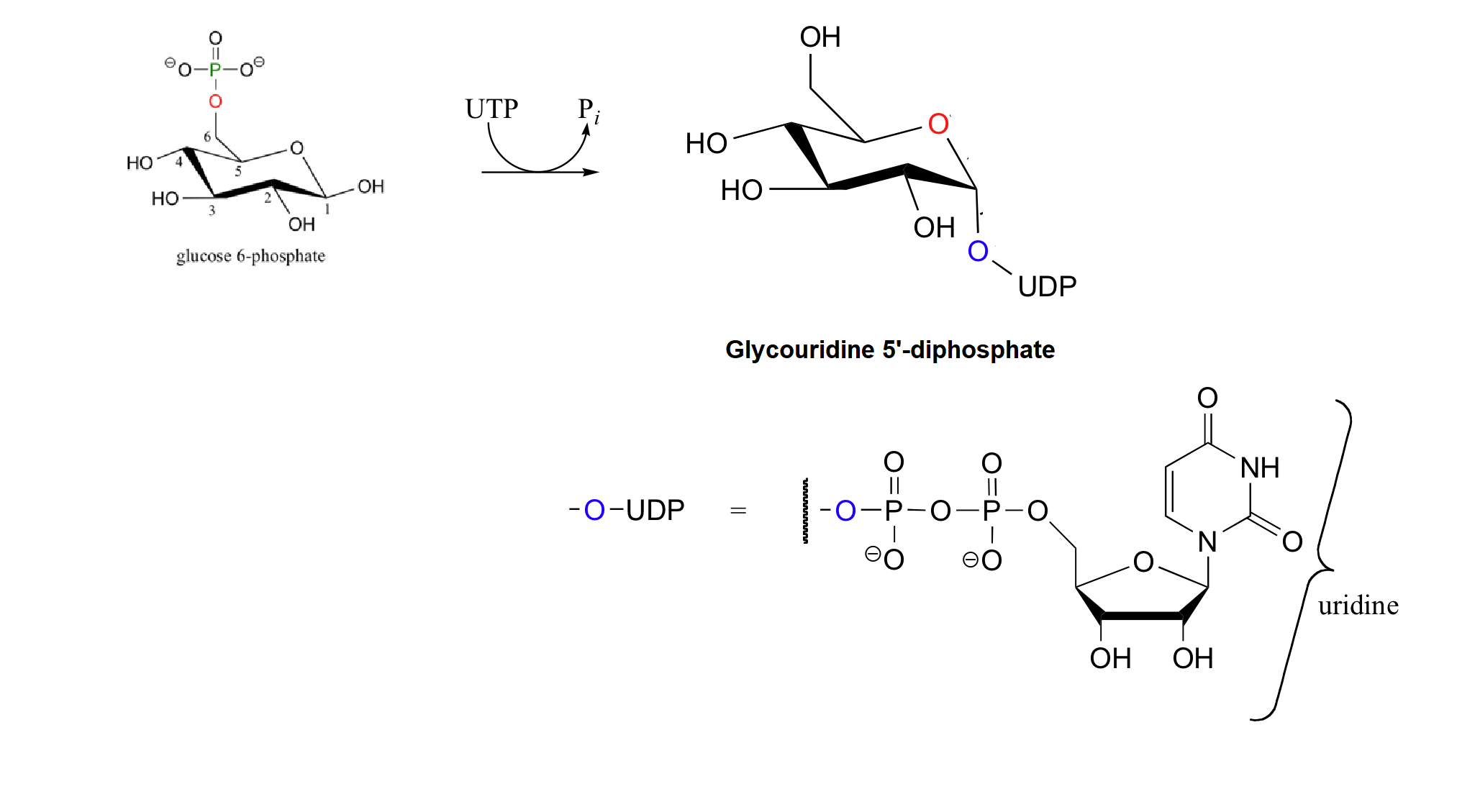
Carbohydrates are covalently attached to many different biomolecules, including lipids, to form glycolipids, and proteins, to form glycoproteins. Called glycoconjugates these sugar bonded molecules are often found in biological membranes, to which they are anchored through covalent bonds. These compounds are crucial to determine to how different cell types recognized one another which will be discussed further in Section 25.11.
Glycoconjugate formation begins with the formation of a phosphorylated sugar such as glucose-6-phosphate which is then reacted with uridine triphosphate (UTP), to give a glycosyl uridine diphosphate. The phosphorylation makes the anomeric -OH of the sugar a better leaving group. During nucleophilic substitutions reaction with a protein or a lipid to form a glycoconjugate the anomeric -OH does not leave as a water molecule, but rather as part of a uridine nucleotide diphosphate group.
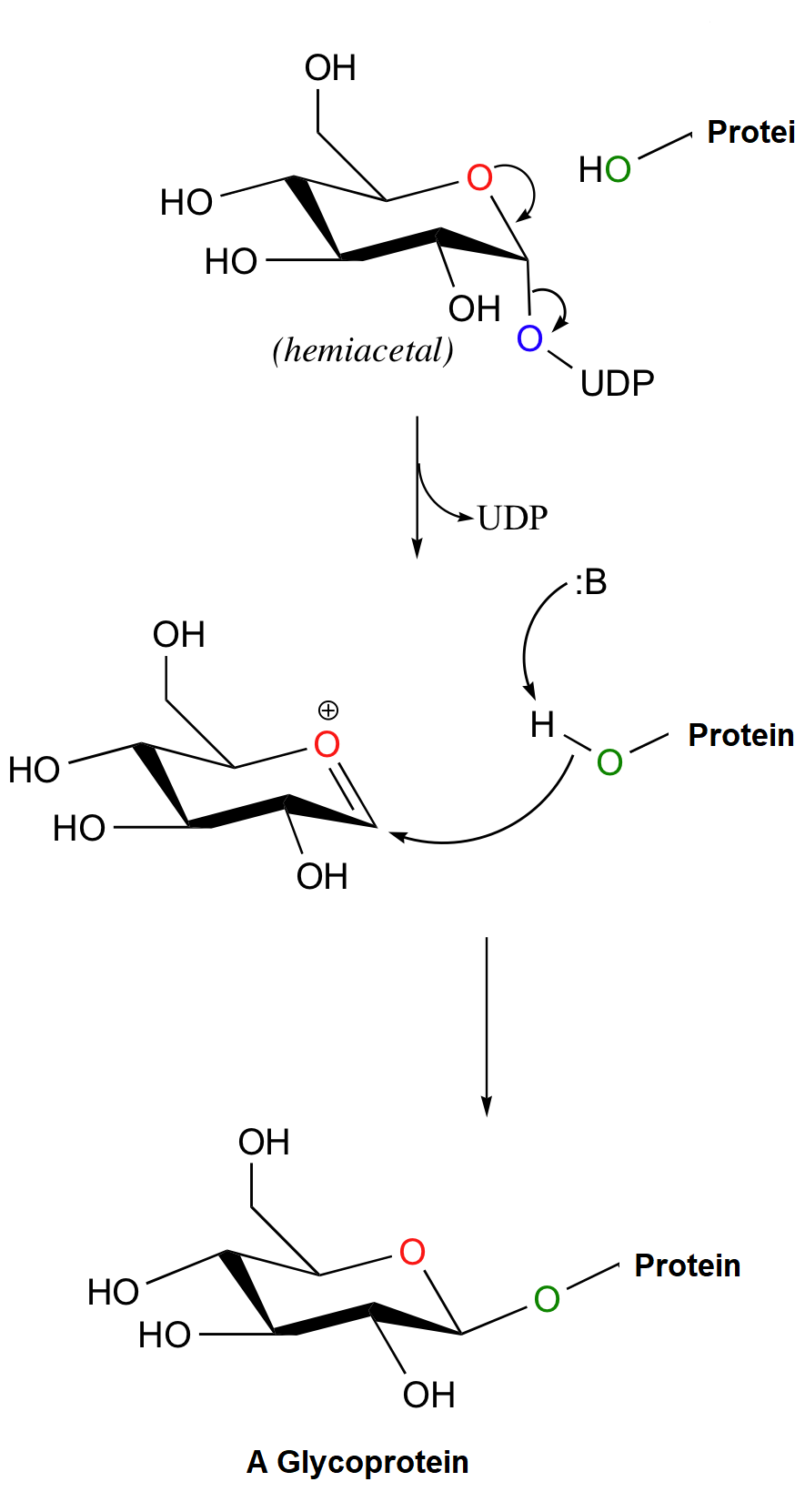
To form a glycoprotein the anomeric carbon of a glucopyranose-UDP derivative is attacked from above by an -OH of -NH2 group from a protein. The UDP leaving group is displaced, and inversion of stereochemistry results at the anomeric carbon.
Oxidation of Monosaccharides
When the aldehyde function of an aldose is oxidized to a carboxylic acid the product is called an aldonic acid. Because of the 2º hydroxyl functions that are also present in these compounds, a mild oxidizing agent such as aqueous Br2 can be used for this conversion. The oxidation occurs by reaction of the open-chain form of the sugar. Because of the equilibrium between the open and ring form of the sugar, eventually the entire sample will undergo the reaction.
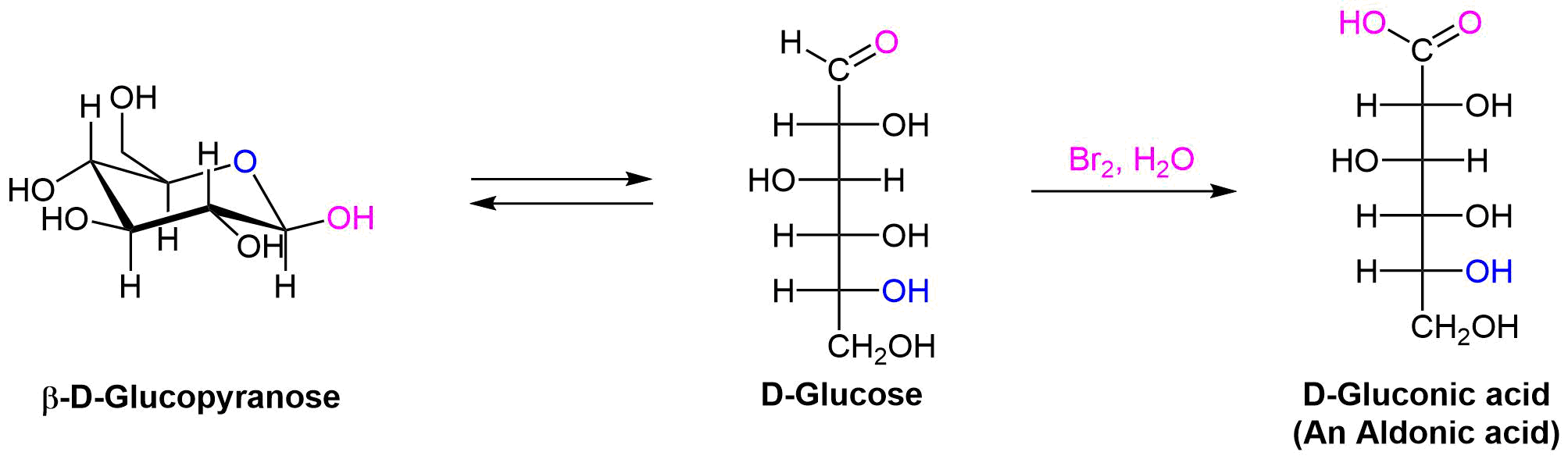
Historically sugars have been classified as reducing or non-reducing based on their reactivity with Tollens' (Ag+ in NH3) or Benedict's (Cu2+ and sodium citrate) reagents. If a sugar is oxidized by these reagents it is called a reducing sugar, since the oxidant (Ag(+) or Cu(+2)) is reduced in the reaction, as evidenced by formation of a silver mirror or precipitation of cuprous oxide. The Tollens' test is commonly used to detect aldehyde functions; and because of the facile interconversion of ketoses and aldoses under the basic conditions of this test, ketoses such as fructose also react and are classified as reducing sugars.
Becuase aldoses contain an aldehyde group they are reducing sugars and will be oxidized by Tollen's and Benedict's reagent. Some ketoses are also reducing sugars. Despite not having an aldehyde groups, fructose is capable of isomerizing to glucose and mannose by keto-enol tautomerism under basic conditions. Once formed, these aldoses are capable of being oxidized by Tollens reagent.
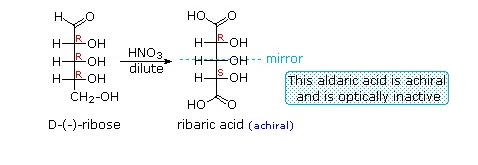
Aldoses can be oxidized to a dicarboxylic acid by dilute nitric acid. Nitric acid is a strong enough oxidizing agent to cause both the aldehyde carbonyl and the -CH2OH group to be oxidized to carboxylic acids. If both ends of an aldose chain are oxidized to carboxylic acids, the product is called an aldaric acid. By converting an aldose to its corresponding aldaric acid derivative, the ends of the chain become identical. Such an operation will disclose any latent symmetry in the remaining molecule and possibly form an achiral meso compound. Thus, ribose, xylose, allose and galactose yield achiral aldaric acids which are, of course, not optically active. The ribose oxidation is shown below.
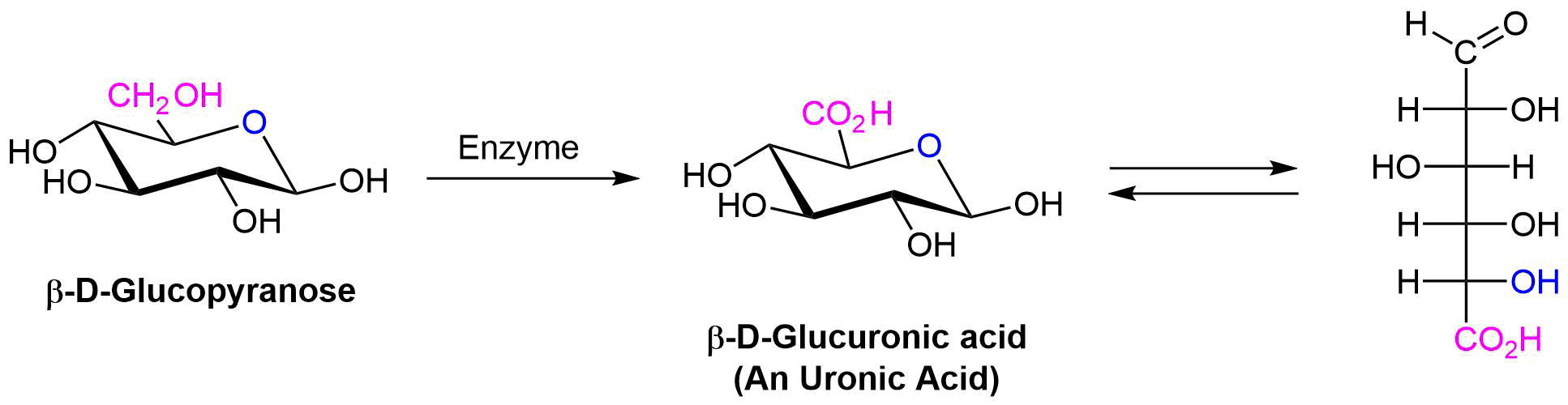
If the -CH2OH group of an aldose is oxidized to a carboxylic acid while the aldehyde carbonyl is not affected, the monocarboxylic acid product is called a uronic acid. This selective oxidation is difficult to accomplish and can only be done enzymatically.
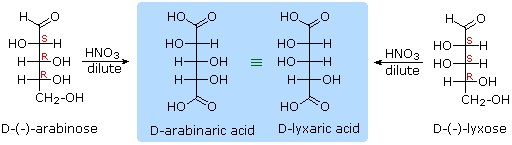
1) D-arabinose and D-lyxose produce the same chiral aldaric acid product when oxidized with dilute HNO3. Please explain. 2) Which two D-aldohexoses are oxidized to produce an optically inactive (meso) aldaric acid? 1) Remember, a Fischer projection formula may be rotated by 180º in the plane of projection without changing its configuration. 2) D-Allose and
Reduction of Monosaccarides
Treatment of an aldose or ketose with sodium borohydride
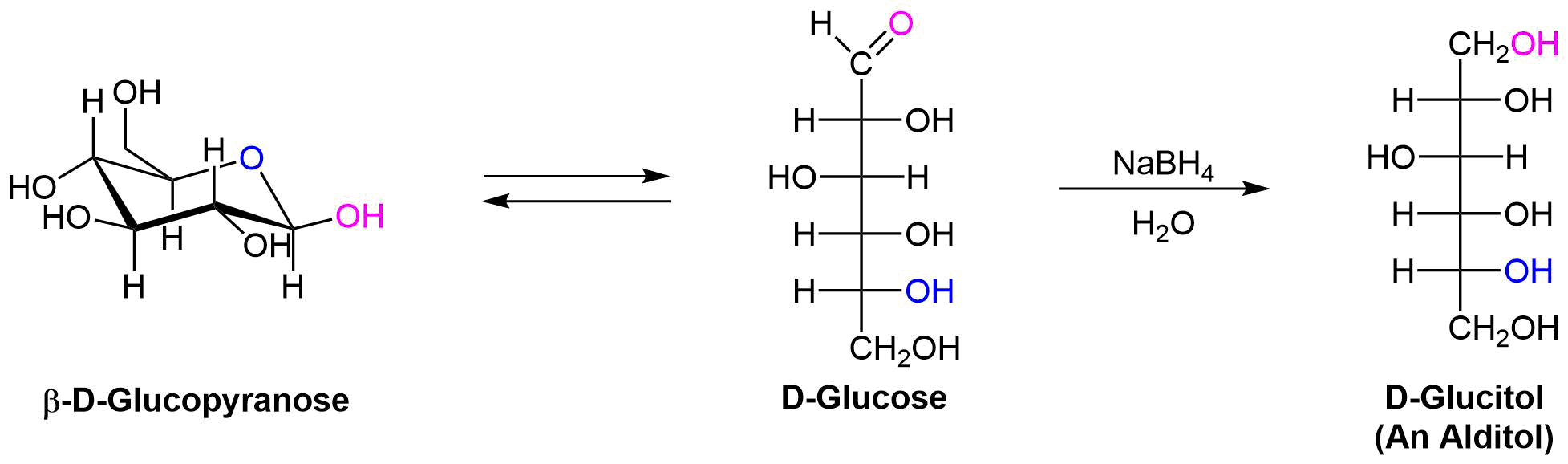
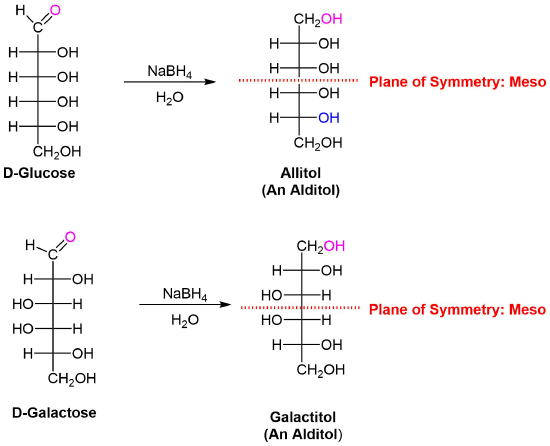
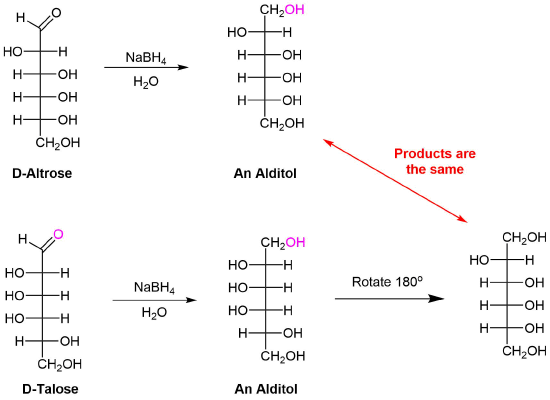
1) Allitol and galactitol from the reduction of allose and galactose are achiral. Explain why this occurs. 2) Altrose and talose are reduced to the same chiral alditol. Explain why this occurs. 1) 2)
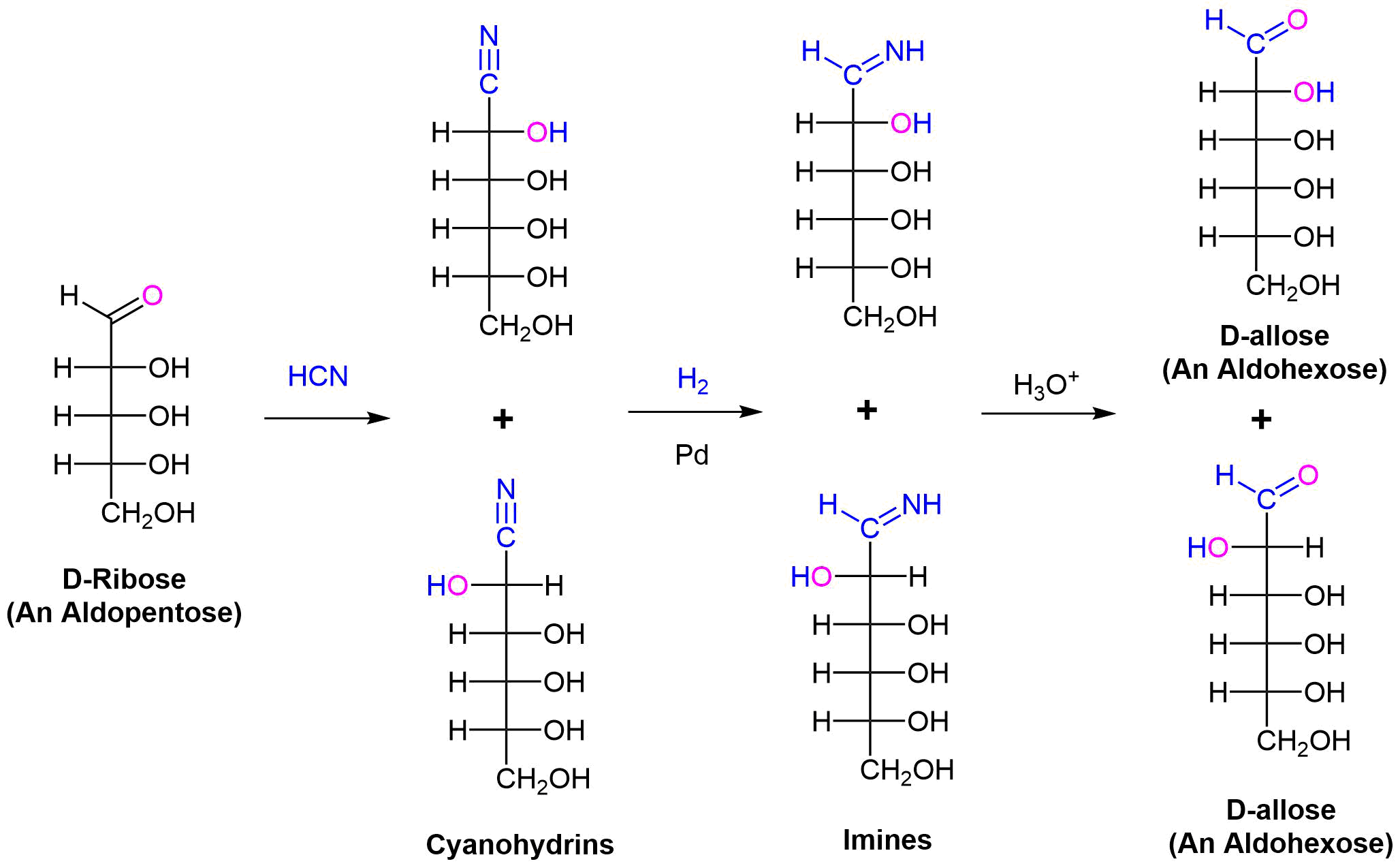
Chain Lengthening: The Kiliani–Fischer Synthesis
The Kiliani–Fischer synthesis lengthens the carbon chain of an aldose by one carbon atom. In doing this two new sugar epimers are created. The synthesis starts by reacting an aldose with HCN. Nucleophilic addition adds the cyanide nucleophile to the electrophilic carbon of the aldose aldehyde forming a cyanohydrin intermediate. The cyanide nucleophile adds a carbon. Stereochemical control is lost, so a racemic mixture of two cyanohydrins differing in the stereochemistry at C2 is formed. The nitrile group of the cyanohydrin is reduced to an imine intermediate by hydrogenation over a palladium catalyst. Finally, the imine is hydrolyzed to an aldehyde to create two new aldoses with an additional carbon. For example performing the Kiliani–Fischer synthesis on D-ribose produces a mixture of D-allose and D-altrose.
1) What products would you expect from Kiliani–Fischer reaction of 2) What aldopentose would be expected to produce a mixture of 1) D-Gluose and D-idose 2) D-Threose
Chain Shortening: The Wohl Degradation
The ability to shorten (degrade) an aldose chain by one carbon was an important tool in the structure elucidation of carbohydrates. This is commonly accomplished the Wohl degradation, which is virtually the reverse of the Kiliani–Fischer synthesis. The aldose aldehyde is converted to an oxime (Section 19-8) by treatment with hydroylamine (NH2OH). Dehydration of the oxime with acetic anhydride forms a cyanohydrin. Under basic conditions, the cyanohydrine loses HCN to reform an aldehyde carbonyl. The following equation illustrates the application of this procedure to the aldopentose, D-arabinose to form the aldotetrose, D-erythrose.
Two of the four D- aldopentoses yield
- Answer
-
D-Ribose and D-arabinose

