Chapter 6 Solutions
- Page ID
- 1121
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
In-chapter exercises
E6.1:
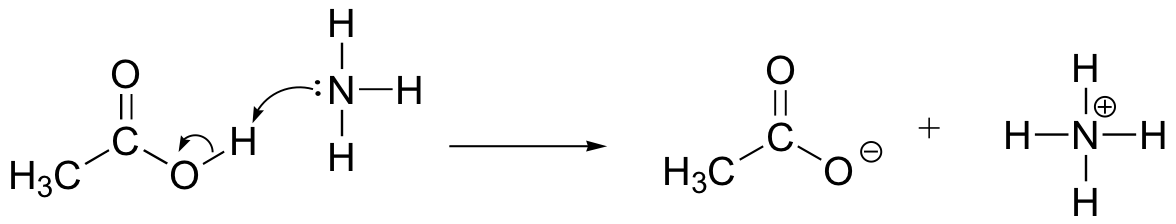
E6.2:
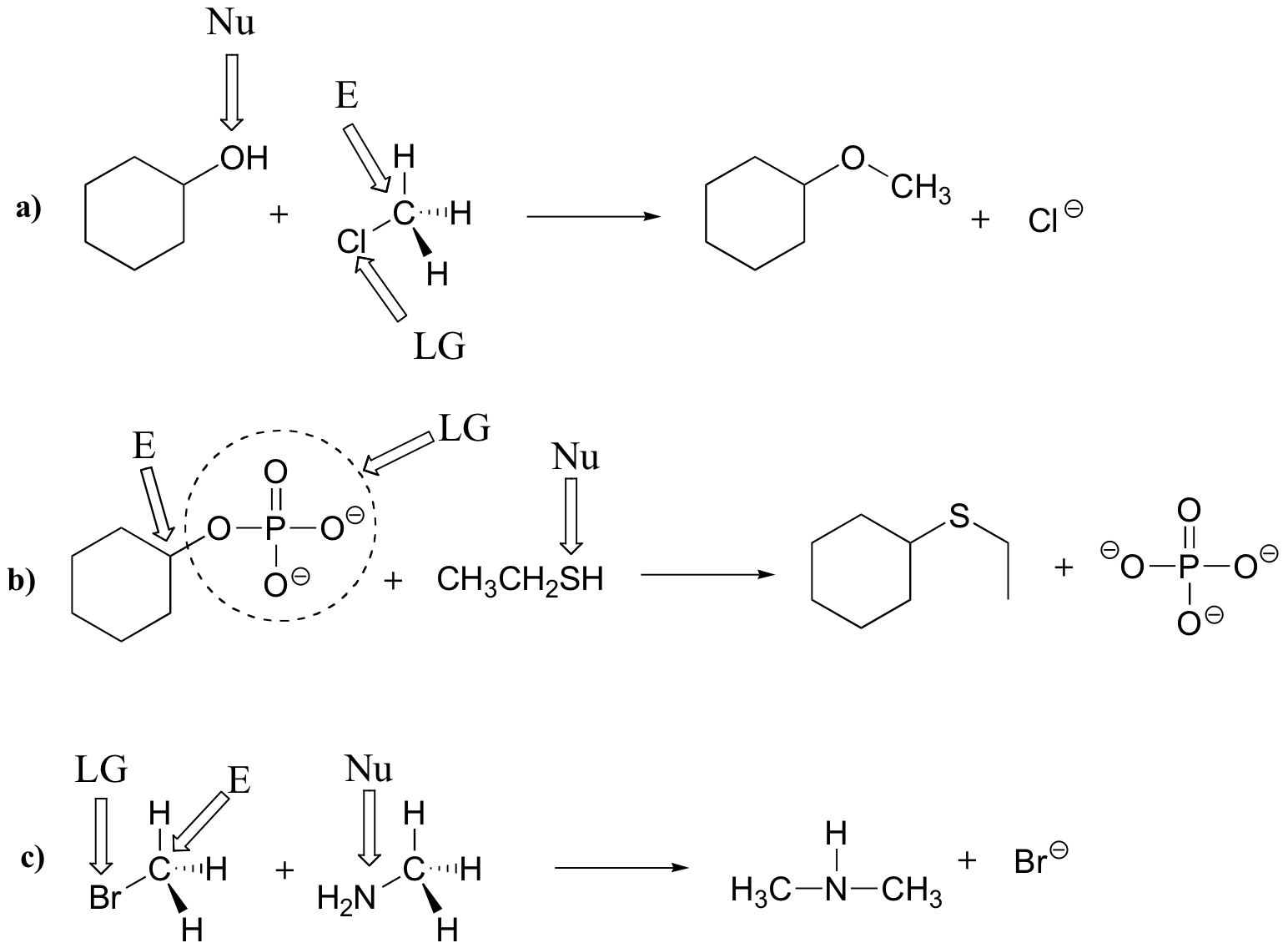
E6.3
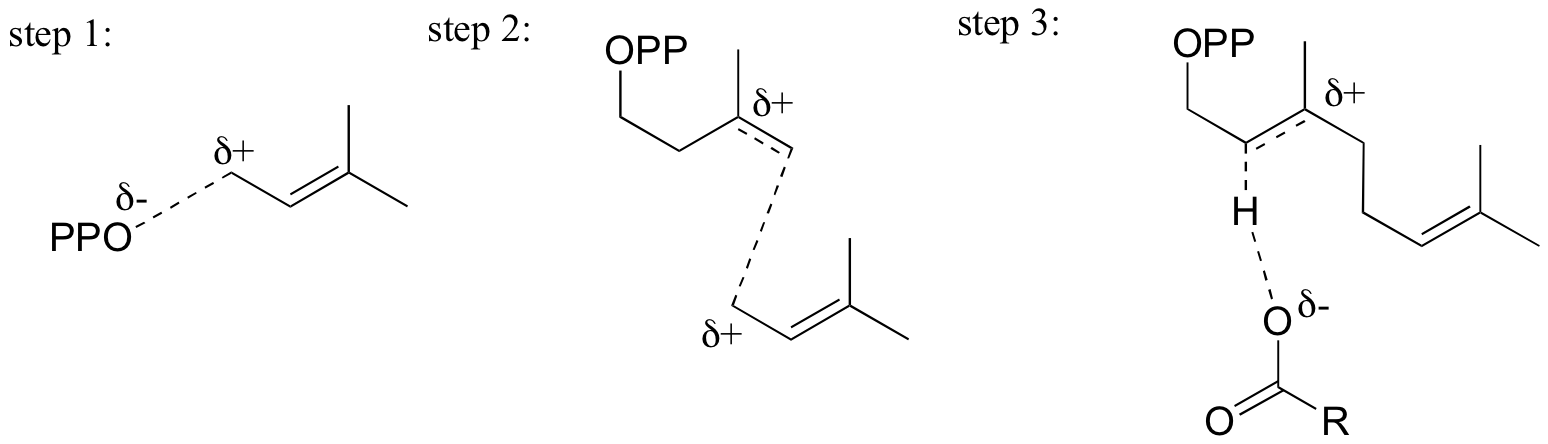
End-of-chapter problems
P6.1:
Left side: the nucleophile is an amine, the electrophile is the methyl carbon, and the leaving group is a sulfide.
Right side: The nucleophile is a thiolate ion, the electrophile is the carbon atom of an alkyl diphosphate, and diphosphate is the leaving group.
P6.2:
a)
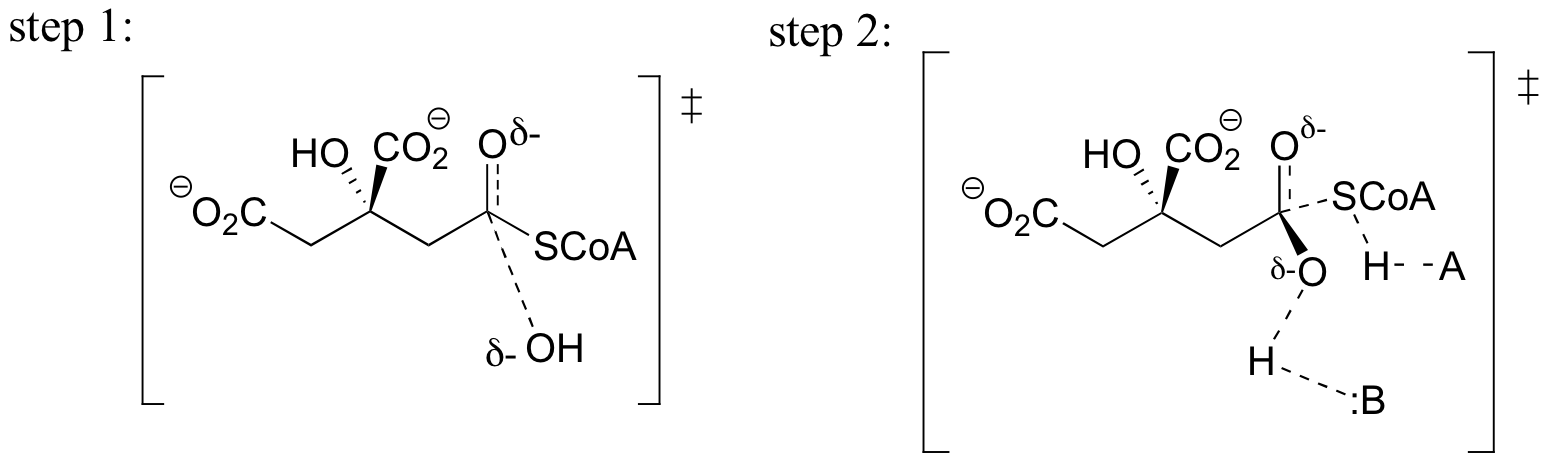
b) In step 1, the nucleophile is the hydroxide oxygen, and the electrophile is the carbonyl carbon of the thioester.
P6.3:
a)
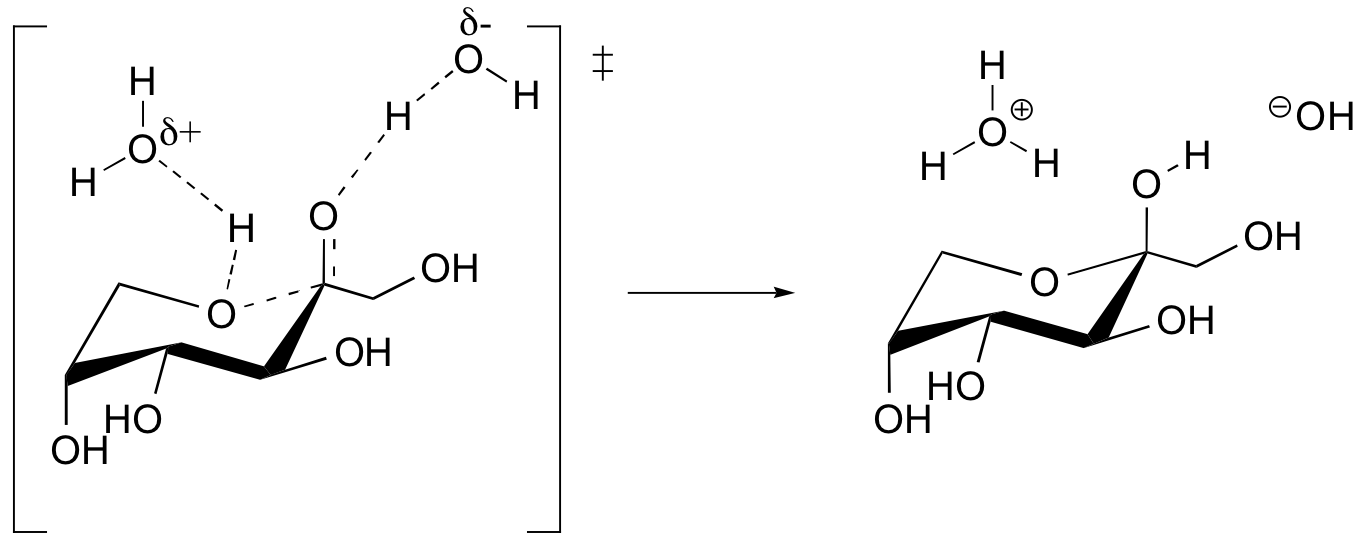
b)
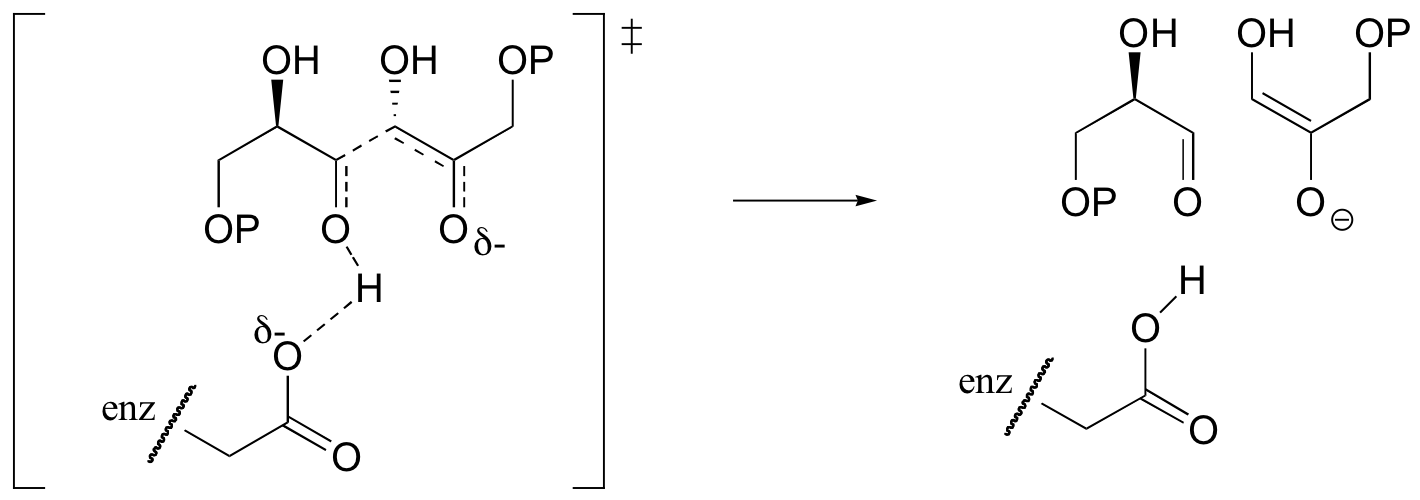
c)
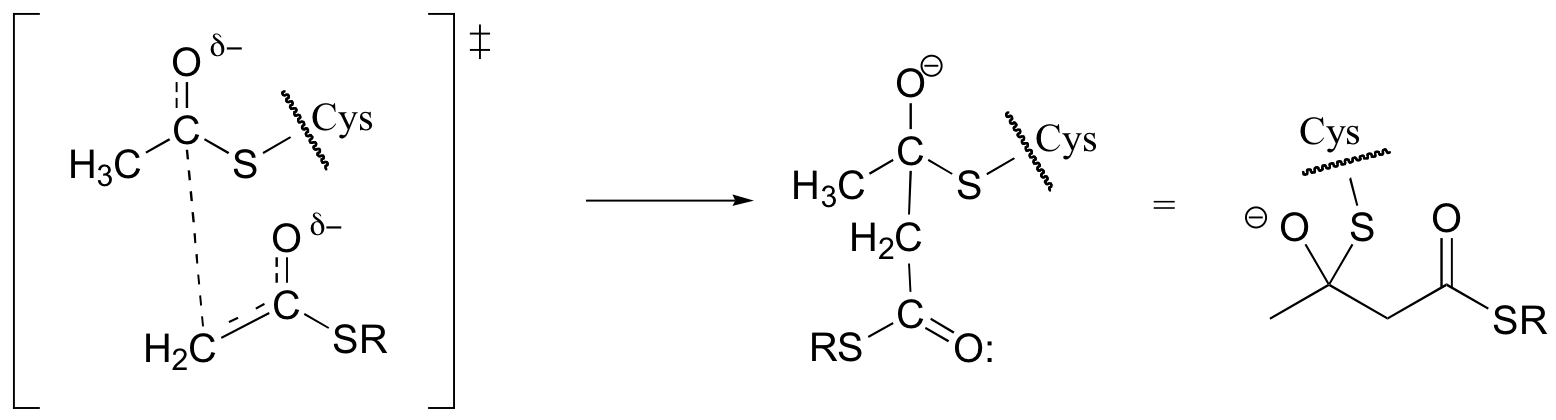
d)
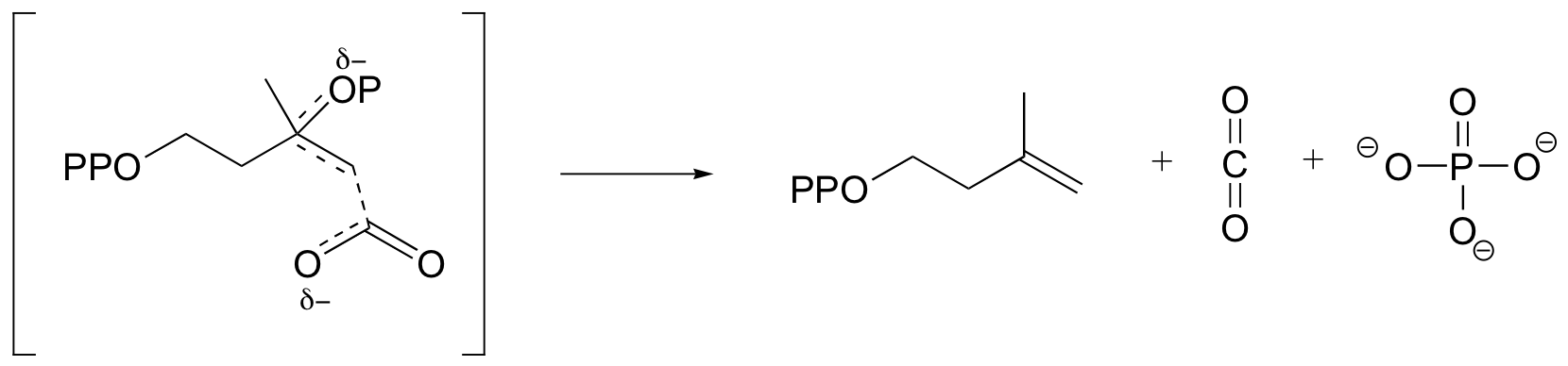
e)
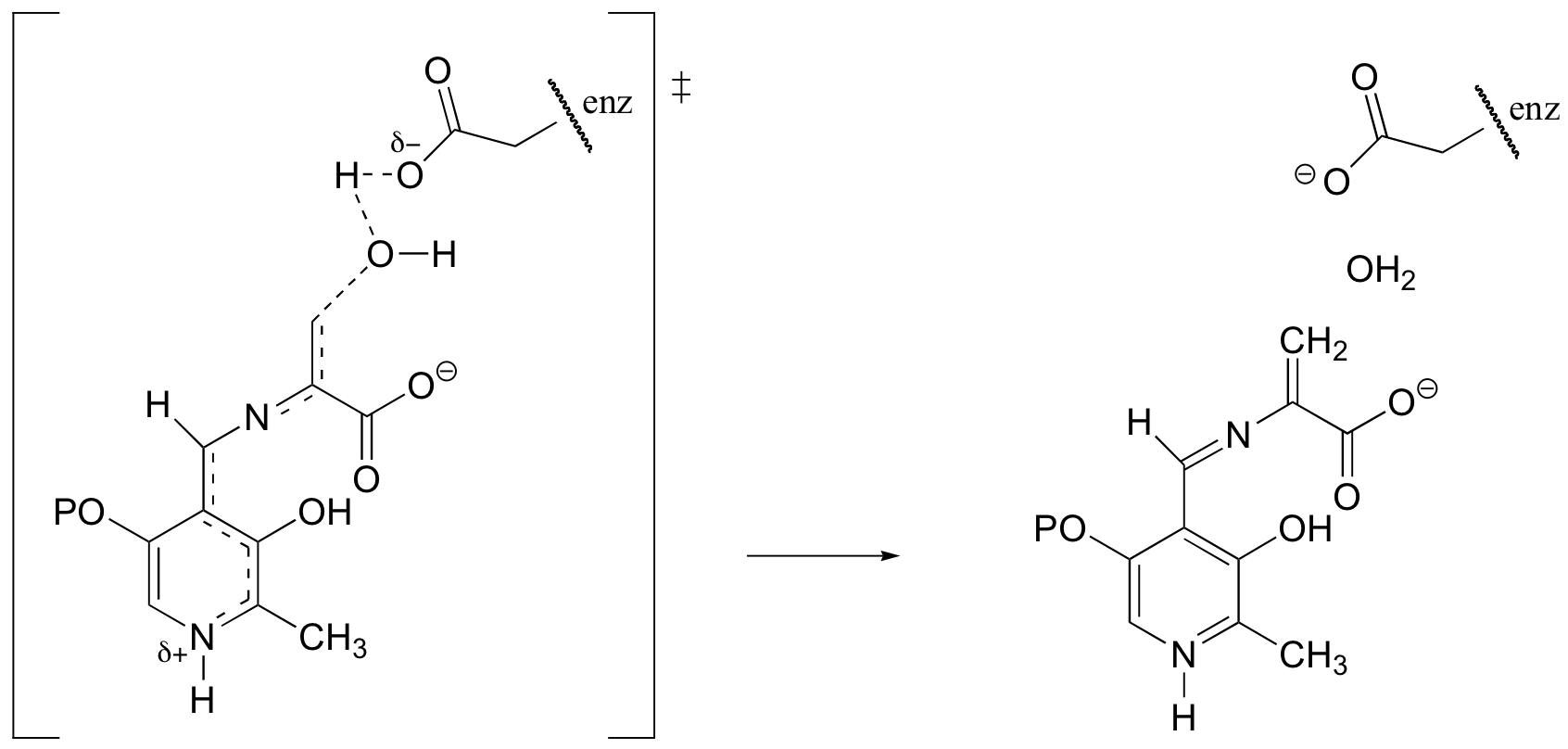
f) Bold dots show the two carbons that form a new bond in this reaction step.
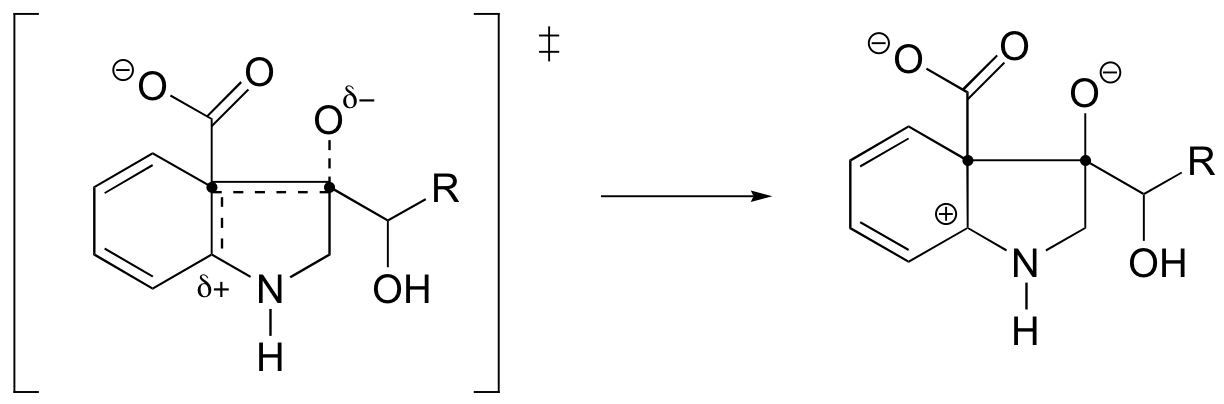
P6.4:
The C to D step has the highest activation energy, and thus is the slowest, rate-determining step.
P6.5:
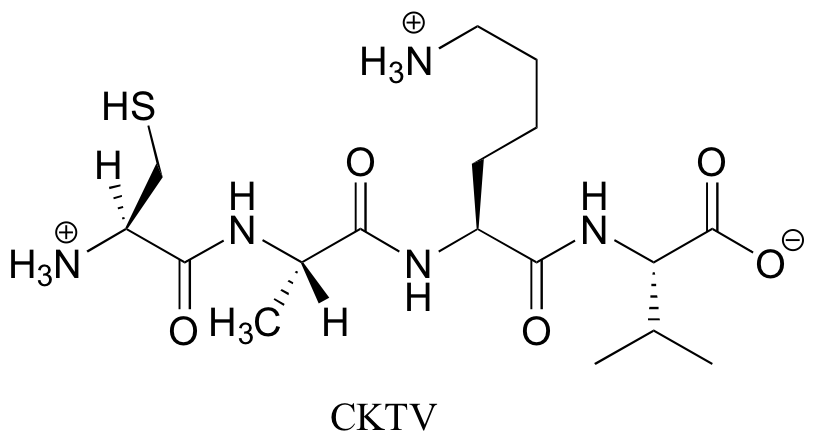
Contributors
- Organic Chemistry With a Biological Emphasis, Tim Soderberg (University of Minnesota, Morris)


