15.1: An overview of the different types of electrophilic reactions
- Page ID
- 994
Electrophilic reactions all involve essentially the same first step: pi electrons attack an electrophile or a proton, forming a carbocation intermediate. Nucleophilic attack by pi electrons on an tetrahedral carbon electrophile can occur in an SN1-like or SN2-like fashion, and pi bonds can also attack carbonyl carbons in what is essentially an electrophilic variation on the carbonyl addition (chapter 11) mechanism.
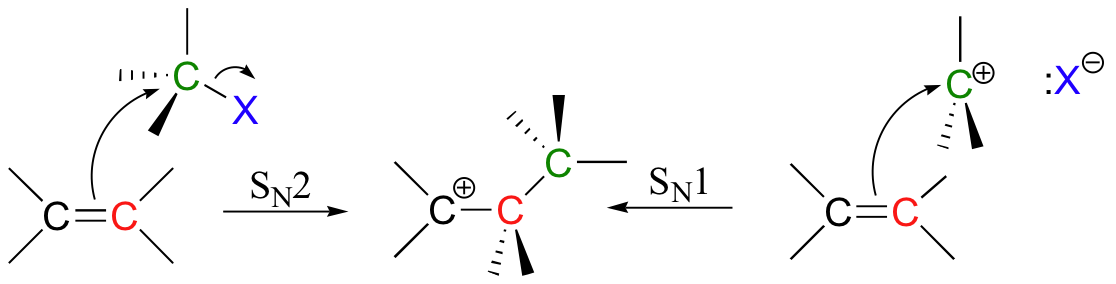
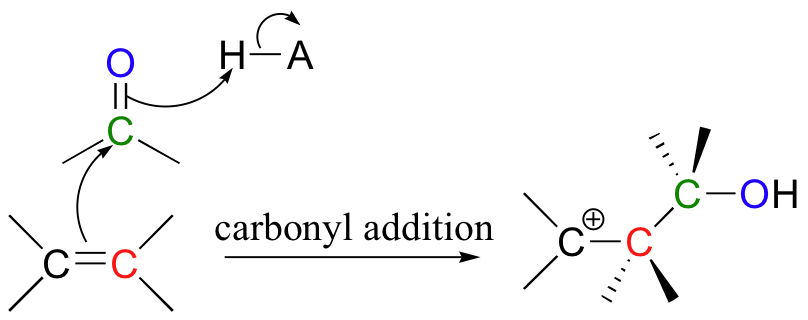
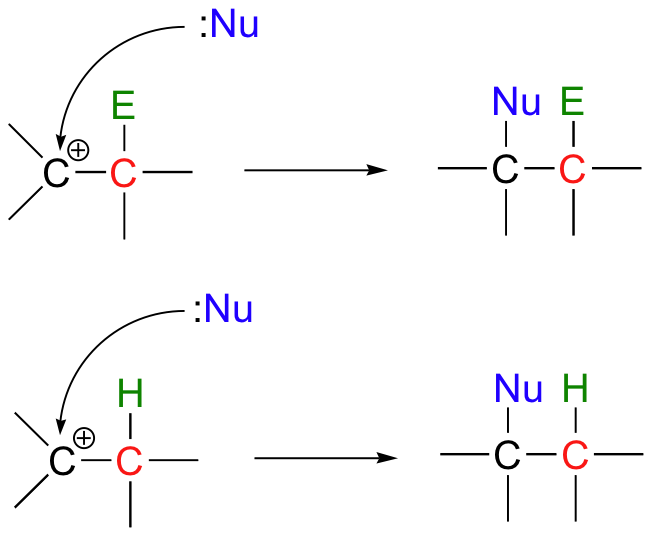
In many cases, a nucleophile next adds to the carbocation intermediate. The result is electrophilic addition (of Nu + E or Nu + H) to the double bond.
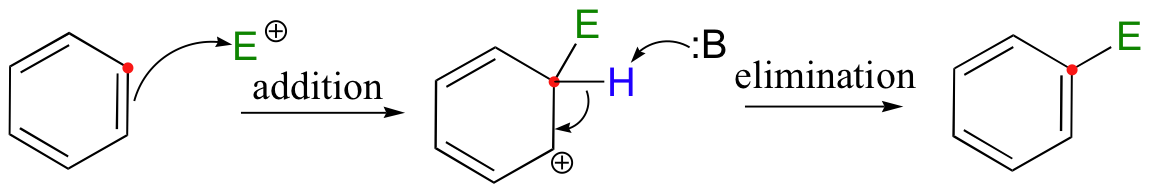
In an electrophilic addition/elimination mechanism, the double bond is preserved. Nucleophilic attack by the pi electrons (addition) is followed by proton abstraction adjacent to the carbocation, leading to re-formation of a double bond (elimination).
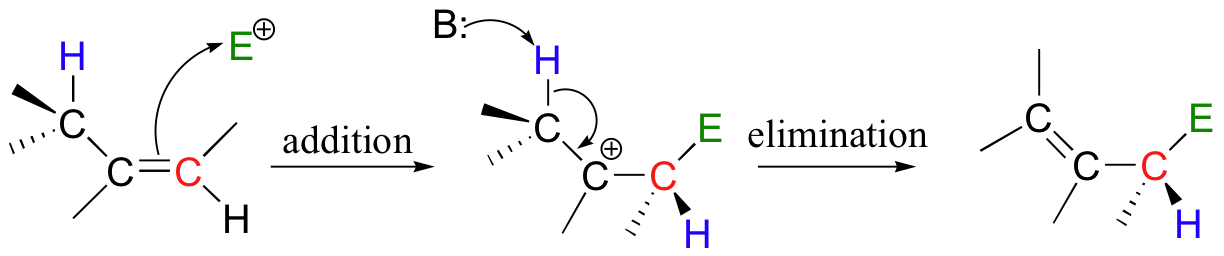
If the pi bond in question is part of an aromatic system, the reaction is referred to as an electrophilic aromatic substitution.