14: Reactions with stabilized carbanion intermediates, part II
- Page ID
- 984
We saw in Chapter 13 how stabilized carbanions - enols, enolated enamines - are key intermediates in biological isomerization reactions and in carbon-carbon bond-forming and bond-breaking events. In this chapter, we will look at two more important reaction types, called Michael additions and β-eliminations, which involve stabilized carbanion species as intermediates. In a Michael addition, a nucleophile and a proton are 'added' to the two carbons of an alkene that is conjugated to a carbonyl. In a beta-elimination, the reverse process occurs:
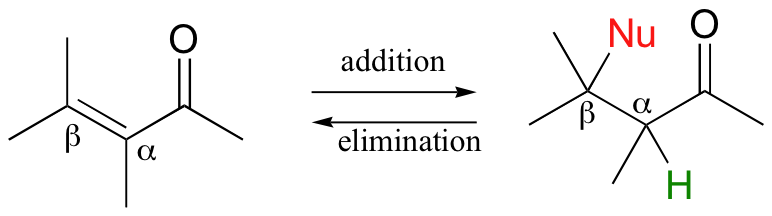
The rich and fascinating chemistry of coenzymes plays a large role in this chapter. We will examine several carbanion-intermediate reactions that involve the participation of pyridoxal phosphate (vitamin B6), a very important carbanion-stabilizing coenzyme. Finally, we will see how the coenzyme thiamine (vitamin B1) allows a carbonyl carbon to act as the nucleophile in a unique class of carbon-carbon bond-forming reaction.


