12: Acyl substitution reactions
- Page ID
- 967
In chapter 11, we learned about the chemistry of aldehydes and ketones. We saw several variations on a single overarching theme – the attack by a nucleophile on the electrophilic carbonyl carbon.
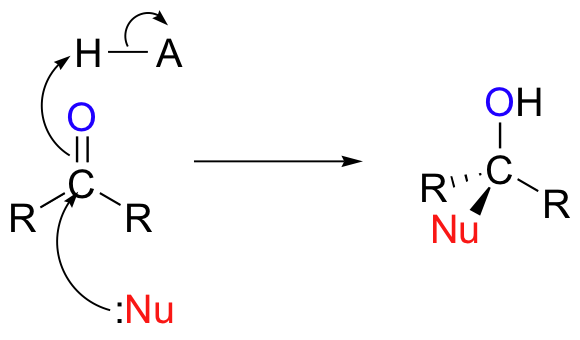
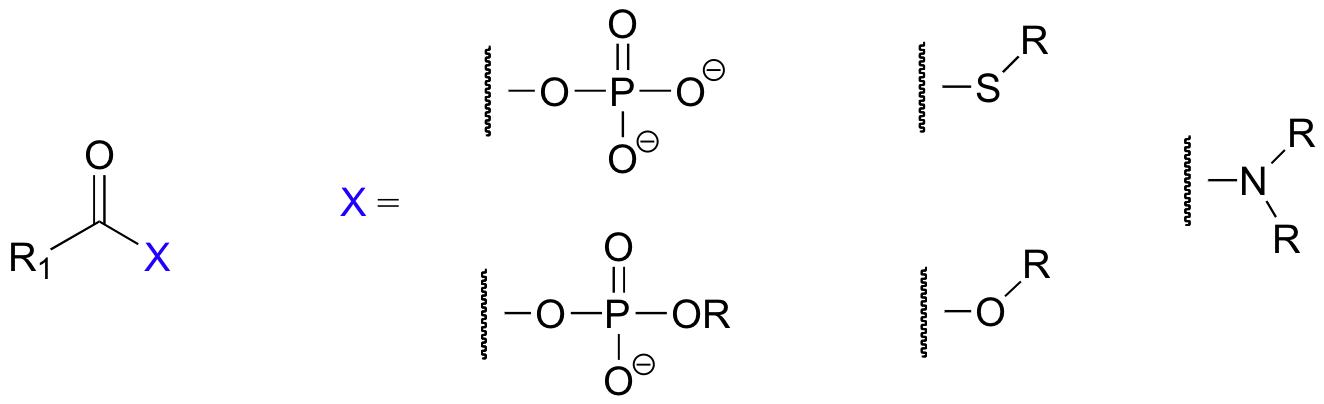
In this chapter, we will see many variations of a reaction type known as nucleophilic acyl substitution, the substrates for which are not aldehydes and ketones but carboxylic acid derivatives, such as acyl phosphates, thioesters, esters, and amides.
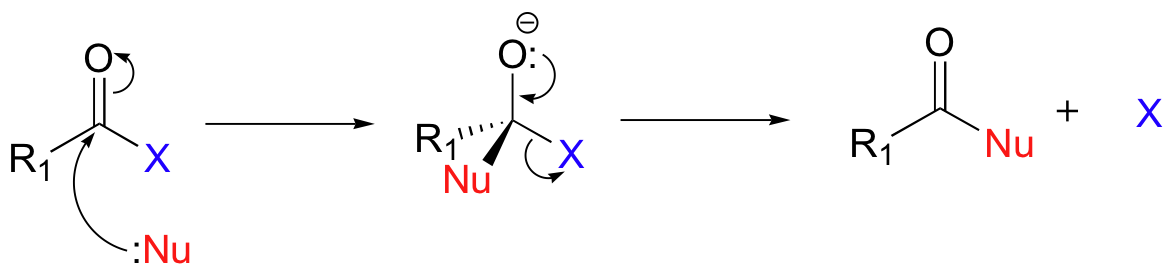
In nucleophilic acyl substitutions, the first step is again the attack of a nucleophile on an electrophilic carbonyl carbon. Because the carbonyl carbon is bonded directly to a leaving group, however, the tetrahedral intermediate quickly collapses, expelling the leaving group (X in the figure below) and re-forming the trigonal planar, sp2-hybridized carbonyl.
It is difficult to overemphasize how widespread nucleophilic acyl substitution reactions are in biochemical pathways: you will see them again and again when you take a course in biochemistry. As we shall soon see, these reactions are especially important in the metabolism of lipids (fats and oils) and in the formation and breakdown of peptide bonds in proteins.


