21.8: More on Stabilization Energies
- Page ID
- 22317
It was shown in Section 21-3 that benzene is \(36\)-\(38 \: \text{kcal}\) more stable than the hypothetical molecule 1,3,5-cyclohexatriene on the basis of the differences between experimental heats of combustion, or hydrogenation, and heats calculated from bond energies. We call this energy difference the stabilization energy (SE) of benzene. We have associated most of this energy difference with \(\pi\)-electron delocalization, which is the delocalization energy (DE). The difference between SE and DE will be small only if our bond-energy tables are reliable and steric and strain effects are small.
The problem with bond energies is that we use bond energies that neglect changes in bond strength caused by environment. Primary, secondary, tertiary, alkenic, and alkynic \(\ce{C_H}\) bonds are assumed to have equal energies; \(\ce{C-C}\) single bonds are assumed to be equal, regardless of whether the other bonds to the carbon atoms in question are single or multiple; and differences in energy between double bonds that are mono-, di-, tri-, or tetra-substituted are neglected, as are changes in bond energies associated with steric strain. Bond energies are strictly applicable to molecules in which the bonds are of the normal lengths. In the case of benzene, which has \(\ce{C-C}\) bonds with lengths intermediate between normal single and double bonds, there seems to be no clear agreement as to how to take the bond distances into account in computing the delocalization energy. In spite of these uncertainties the stabilization energies seem to give a good qualitative idea of the importance of electron delocalization in organic molecules.
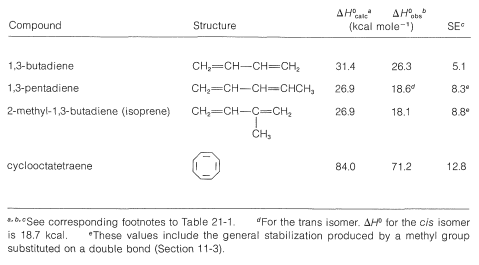
Tables 21-1 and 21-2 give stabilization energies for several substances that are best represented as hybrid structures.
Table 21-1: Stabilization Energies (or Approximate Delocalization Energies) from Heats of Formation of Some Aromatic Compounds
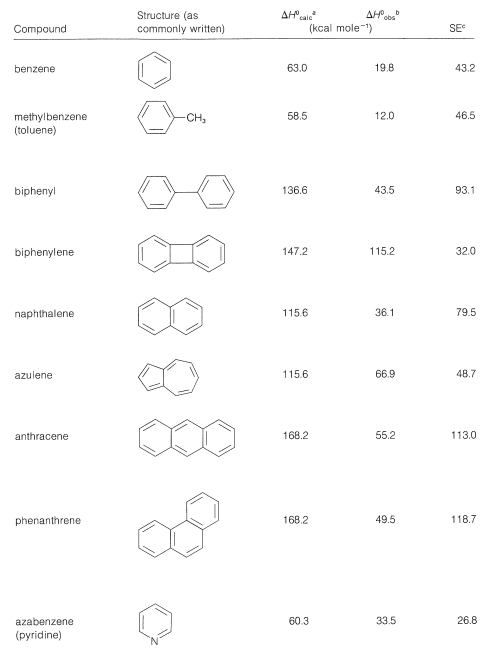
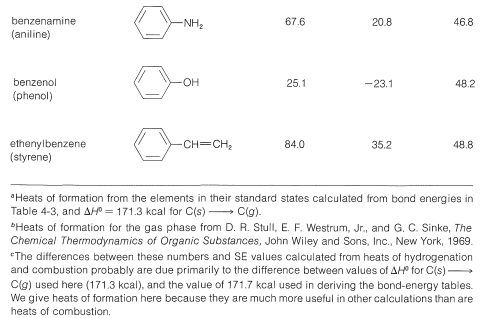
Table 21-2: Stabilization Energies (SE) from Heats of Formation \(\left( \Delta H^0 \right)\) of Some Conjugated Polyenes
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


