20.6: Glycosides
- Page ID
- 22305
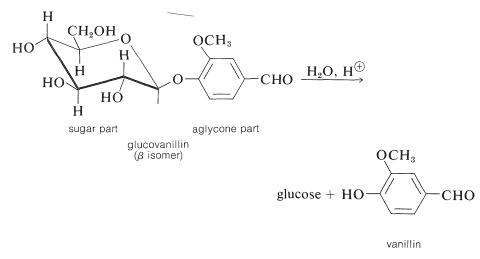
Although abundant quantities of glucose and fructose are found in the free state, they and less common sugars occur widely in plants and animals combined with various hydroxy compounds. The bonding is through oxygen to the carbonyl carbon, as in the \(\alpha\)- and \(\beta\)-methylglucosides discussed in Section 20-4A, to give acetal or ketal structures. These substances are sometimes simply called glycosides, but it is desirable to specify that the bonding is through oxygen by using the name \(\ce{O}\)-glycoside. Hydrolysis of an \(\ce{O}\)-glycoside gives the sugar and the hydroxy compound, called the aglycone component of the glycoside.
A specific example is glucovanillin, which can be isolated from the green fruit pods of vanilla, a climbing orchid cultivated in several tropical countries. Hydrolysis gives glucose and the aglycone, vanillin, which is the principal ingredient of vanilla flavoring. As the vanilla pods mature, a natural hydrolysis reaction proceeds to the extent that the pods may be covered with small crystals of vanillin.
The configurations of glycosides are designated by the same convention used for the sugar anomers. Thus if a glycoside of a \(D\) sugar has the \(D\) configuration at the anomeric carbon, it is designated as the \(\alpha\)-\(D\)-glycoside, and if it has the \(L\) configuration it is called the \(\beta\)-\(D\)-glycoside (see Section 20-3). If the sugar involved in glycoside formation is glucose, the derivative is a glucoside; if fructose, a fructoside; if galactose, a galactoside; and so on. When the hydroxy compound, or aglycone, is another sugar, then the glycoside is a disaccharide, and if the sugar is already a dissacharide, the glycoside is a trisaccharide, and so on.
Among the natural products that occur as glycosides (most commonly as \(\beta\)-\(D\)-glucosides) are many plant pigments (the anthocyanins), the flavorings vanillin and amygdalin, and many steroids (such as the cardiac glycosides and saponins). The structures of some of these substances will be discussed in later chapters.
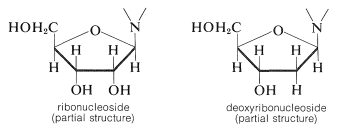
Not all glycosides are \(\ce{O}\)-glycosides. A group of \(\ce{N}\)-glycosides of special biological importance are derived from heterocyclic nitrogen bases and \(D\)-ribose and 2-deoxy-\(D\)-ribose. They commonly are known as nucleosides, or more specifically, as ribonucleosides and deoxyribonucleosides; the \(\ce{N}\)-glycoside linkage is always \(\beta\):
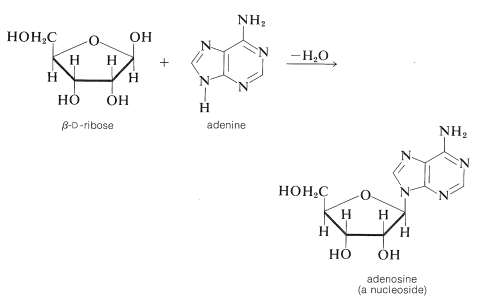
The \(\ce{N}\)-glycoside of \(D\)-ribose and the nitrogen heterocycle, adenine, is adenosine:
A nucleotide is a phosphate ester of a nucleoside. The hydroxyl group at the \(\ce{C_5}\) position of the pentose is the most common site of esterification. The nucleotides of adenosine are ATP, ADP, and AMP (Section 15-5F).
A dinucleotide is a combination of two nucleosides through a common phosphate ester link. Familiar examples are \(\ce{NAD}^\oplus\), \(\ce{NADH}\), \(\ce{FAD}\), and \(\ce{FADH_2}\) (Section 15-6C). Polynucleotides are polymers of nucleosides linked through phosphate ester bonds. Polynucleotides also are called nucleic acids (RNA and DNA) and are the genetic material of cells, as will be discussed in Chapter 25.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


