19.8: Prochirality
- Page ID
- 22780
In the transformations shown in Equations 19-5 and 19-6, the organic reactants are symmetrical molecules (with no chiral centers), but the products are asymmetric molecules (each has a chiral carbon):
.jpg?revision=1) \(\tag{19-5}\)
\(\tag{19-5}\)
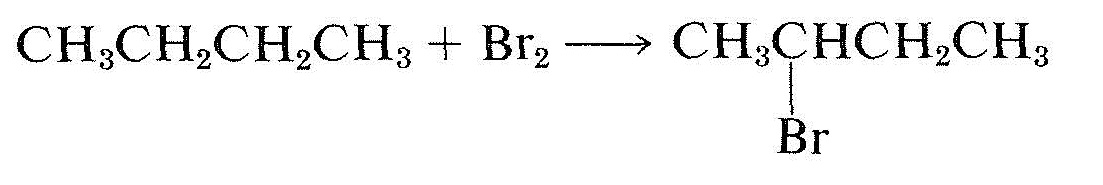 \(\tag{19-6}\)
\(\tag{19-6}\)
There is a special term for molecules that are achiral but which can be converted to molecules with chiral centers by a single chemical substitution or addition reaction. They are said to be prochiral.
By this definition, ethanol is a prochiral molecule. The two methylene hydrogens are enantiotopic (Section 9-10C) and substituting each separately (with, say, one deuterium) leads to a pair of enantiomers:
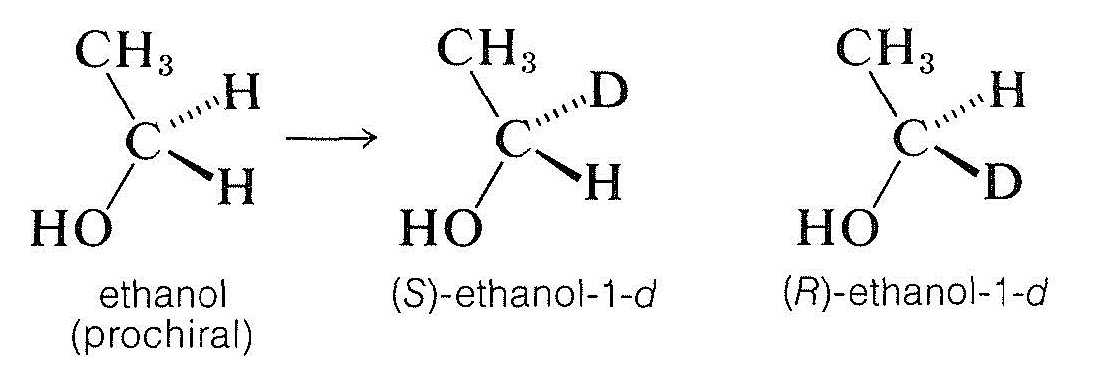
Prochiral molecules can be distinguished readily from more symmetrical molecules because they lack a two-fold symmetry axis passing through the prochiral center, as the following rotations show:
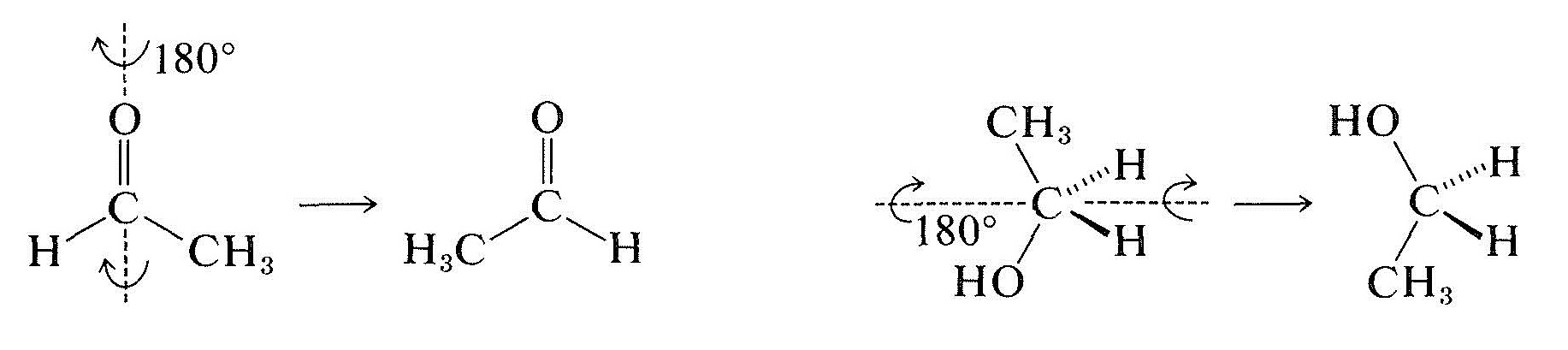
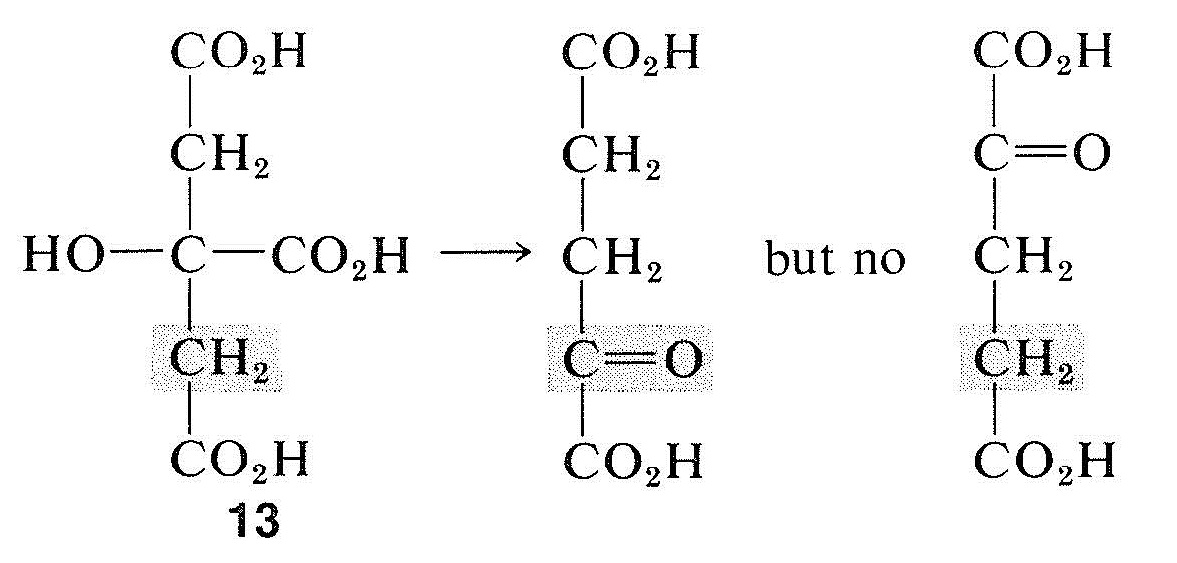
Organic chemists have not had much use for prochirality, but it is an important concept for biochemists following the stereochemistry of bio-organic reactions. Almost all biochemical reactions are under the control of enzymes, which function asymmetrically even on symmetrical (but prochiral) molecules. Thus it has been found that only one of the two methylene groups of citric acid, \(13\), is converted by enzymes (from rat liver) to the carbonyl of 2-oxobutanedioic acid:
The notation prochiral center is useful in molecules that already have one or more chiral centers. Development of chirality from prochirality in such cases would lead to diastereomers, as shown in the conversions of \(14\) and \(15\):

Contributors
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


