19.6: The R,S Convention for Designating Stereochemical Configurations
- Page ID
- 22762
There are certain disadvantages to the \(D\),\(L\) system using Fischer projection formulas to denote configuration about a chiral center, and we already have seen how ambiguity arises in the case of the tartaric acids (Section 19-5). A more systematic way of denoting configuration that may eventually replace the \(D\),\(L\) system, at least for simple compounds, is known as the \(R\),\(S\) or Cahn-Ingold-Prelog convention, after its originators.
To denote the configuration of a chiral center by the \(R\),\(S\) convention, the groups at the center are assigned an order of precedence according to a specific set of rules based on atomic numbers. Suppose a carbon atom is bonded to four different substituents, which we will designate \(A\),\(B\), \(C\), and \(D\) and to which we assign the following priority sequences: \(A\) before \(B\) before \(C\) before \(D\). If we now view the arrangement of \(A\), \(B\), and \(C\) from the site remote from the substituent of lowest priority, \(D\), as shown in Figure 19-6, and the sequence turns out to be \(A \rightarrow B \rightarrow C\) in the clockwise direction, then the configuration is said to be \(R\). If the sequence \(A \rightarrow B \rightarrow C\) occurs in the counterclockwise direction, the configuration is \(S\). The symbols \(R\) and \(S\) are taken from the Latin words rectus and sinister, meaning right and left, respectively.
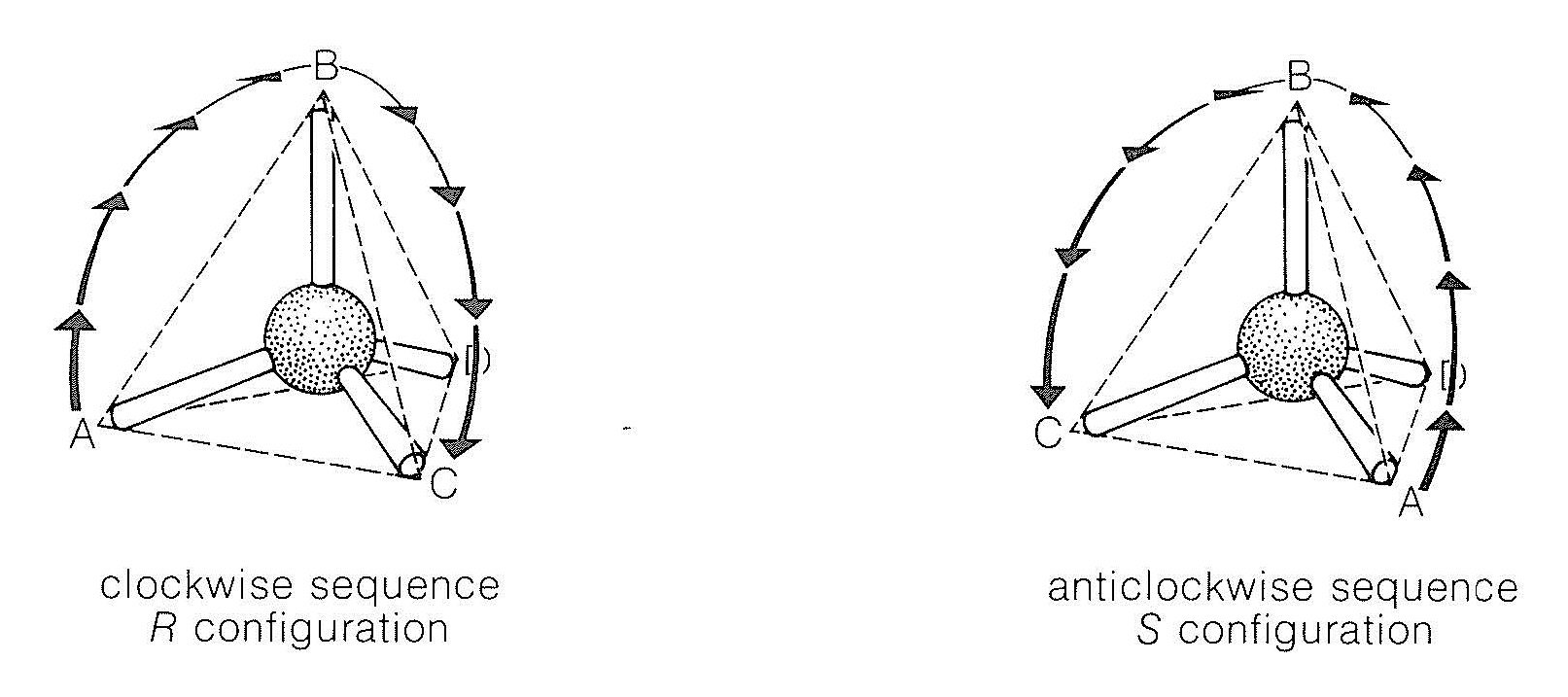
The understanding of \(R\) and \(S\) is simple; the problems are in assigning the priority sequences for actual substituents. The rules follow:
1. Priority is given to the substituent atoms that have the highest atomic number. This means that four different atoms arranged tetrahedrally about the chiral center have a priority sequence that decreases with decreasing atomic number. For example, the sequence among the halogens is \(\ce{I} > \ce{Br} > \ce{Cl} > \ce{F}\), and Structure \(5\) (shown here in perspective and in projection) therefore has the \(R\) configuration:
.jpg?revision=1)
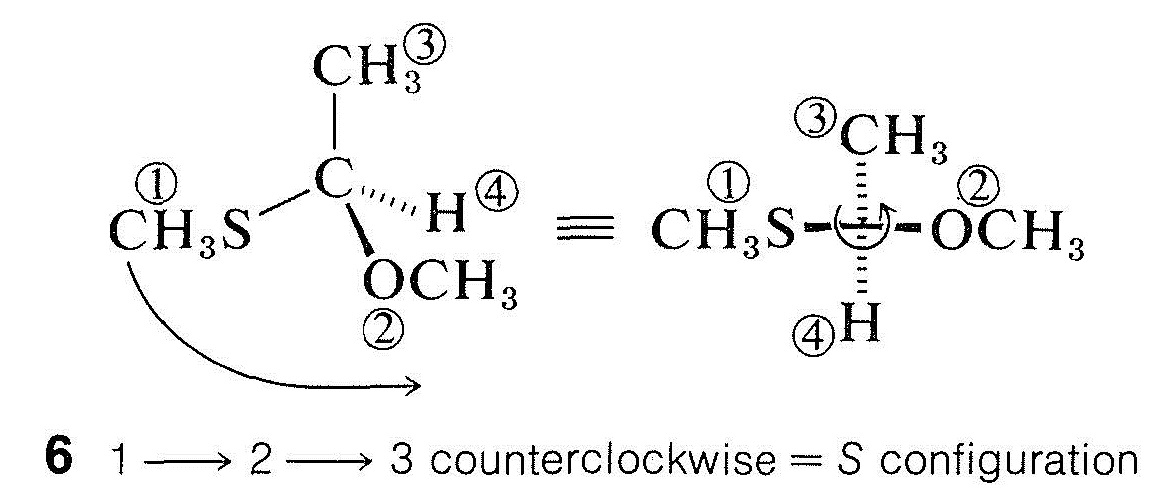
For more complex substituents, priority is determined by the atomic number of the first bonded atom. The sequence \(\ce{CH_3S} > \ce{CH_3O} > \ce{NH_2} > \ce{CH_3} > \ce{H}\) thus reflects the fact that atomic number decreases in the order \(\ce{S} > \ce{O} > \ce{N} > \ce{C} > \ce{H}\). Structure \(6\) accordingly has the \(S\) configuration:
2. The first atoms in two or more substituents often are identical, in which case it is necessary to explore further and compare the atomic numbers of the second attached atoms. Precedence is given to the substituent with a second atom of higher atomic number. For example, in 2-butanol, \(\ce{CH_3CH(OH)CH_2CH_3}\), two of the groups at the chiral atom have carbon as the first atom. We therefore must compare the other atoms bonded to these two carbons. It is convenient to represent the arrangement at the chiral atom as shown in \(7\), where the first atoms are shown attached to the chiral center and the second atoms are listed in their priority order; thus, \(\left( \ce{C}, \: \ce{H}, \: \ce{H} \right)\) for ethyl and \(\left( \ce{H}, \: \ce{H}, \: \ce{H} \right)\) for methyl:
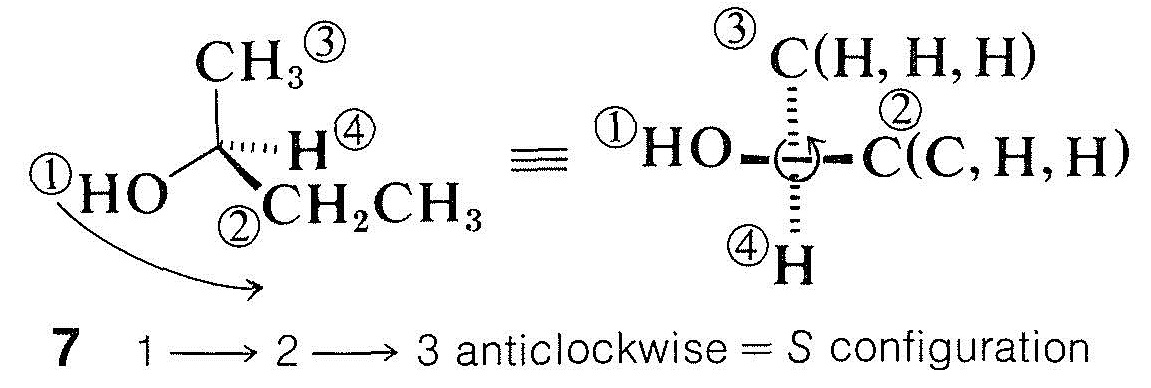
When we compare \(\left( \ce{H}, \: \ce{H}, \: \ce{H} \right)\) with \(\left( \ce{C}, \: \ce{H}, \: \ce{H} \right)\) in \(7\), we give ethyl precedence over methyl because carbon has a higher atomic number than hydrogen. The configuration \(7\) therefore must be \(S\).
3. Double and triple bonds are treated as if they had duplicate or triplicate single bonds. Thus a carbonyl group, _-_Copy.jpg?revision=1) , is treated as if it were
, is treated as if it were .jpg?revision=1) where the symbols in parentheses represent the duplicate atoms.
where the symbols in parentheses represent the duplicate atoms.
Let us see how this works for 3-chloro-1-pentyne, \(8\):
.jpg?revision=1)
The first-atom priority sequence is \(\ce{Cl} > \ce{C}\) and \(\ce{C} > \ce{H}\). We now need to order \(\ce{-CH_2CH_3}\) and \(\ce{-C \equiv CH}\) and, in doing this, we compare the three atoms attached to the first carbon of the ethyl group \(\left( \ce{C}, \: \ce{H}, \: \ce{H} \right)\) with the three attached to the first carbon of the ethynyl group \(\left[ \ce{C}, \: \left( \ce{C} \right), \: \left( \ce{C} \right) \right]\). On this basis, ethynyl comes ahead of ethyl, and the overall sequence is \(\ce{Cl} > \ce{C \equiv CH} > \ce{CH_2CH_3} > \ce{H}\), so \(8\) will have the \(S\) configuration.
The sequence rules described thus far can be used without ambiguity in most of the examples we are likely to meet. The important thing to remember is to look at the kind of atoms attached as far out as necessary. Suppose we have to compare the aldehyde group, \(\ce{-CH=O}\), with the dimethoxymethyl group, \(\ce{CH(OCH_3)_2}\). The first atoms are the same \(\left( \ce{C} \right)\), the second atoms are the same \(\left[ \ce{O}, \: \left( \ce{O} \right), \: \ce{H} \right]\), and the difference arrives at the third-atom level where we are comparing lone pairs (priority zero) with carbons. Thus \(\ce{-CH(OCH_3)_2}\), outranks \(\ce{-CH=O}\).
Comparison of groups such as isopropyl and ethenyl is more difficult and requires knowing what the convention is when we have to go to the far end of a double bond. A useful way of writing these groups is as follows:
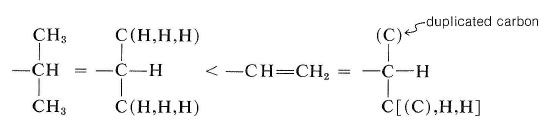
We put ethenyl ahead of isopropyl because \(\left[ \left( \ce{C} \right), \: \ce{H}, \: \ce{H} \right]\) takes priority over \(\left( \ce{H}, \: \ce{H}, \: \ce{H} \right)\). It is important to understand that the nonduplicated carbon is considered to be connected to the duplicated carbon as well as the two hydrogens in arriving at the connection pattern \(\left( \left( \ce{C} \right), \: \ce{H}, \: \ce{H} \right)\).
The same kind of logic leads to the following sequence:
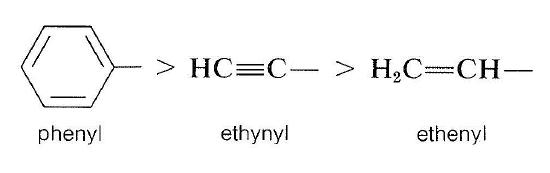
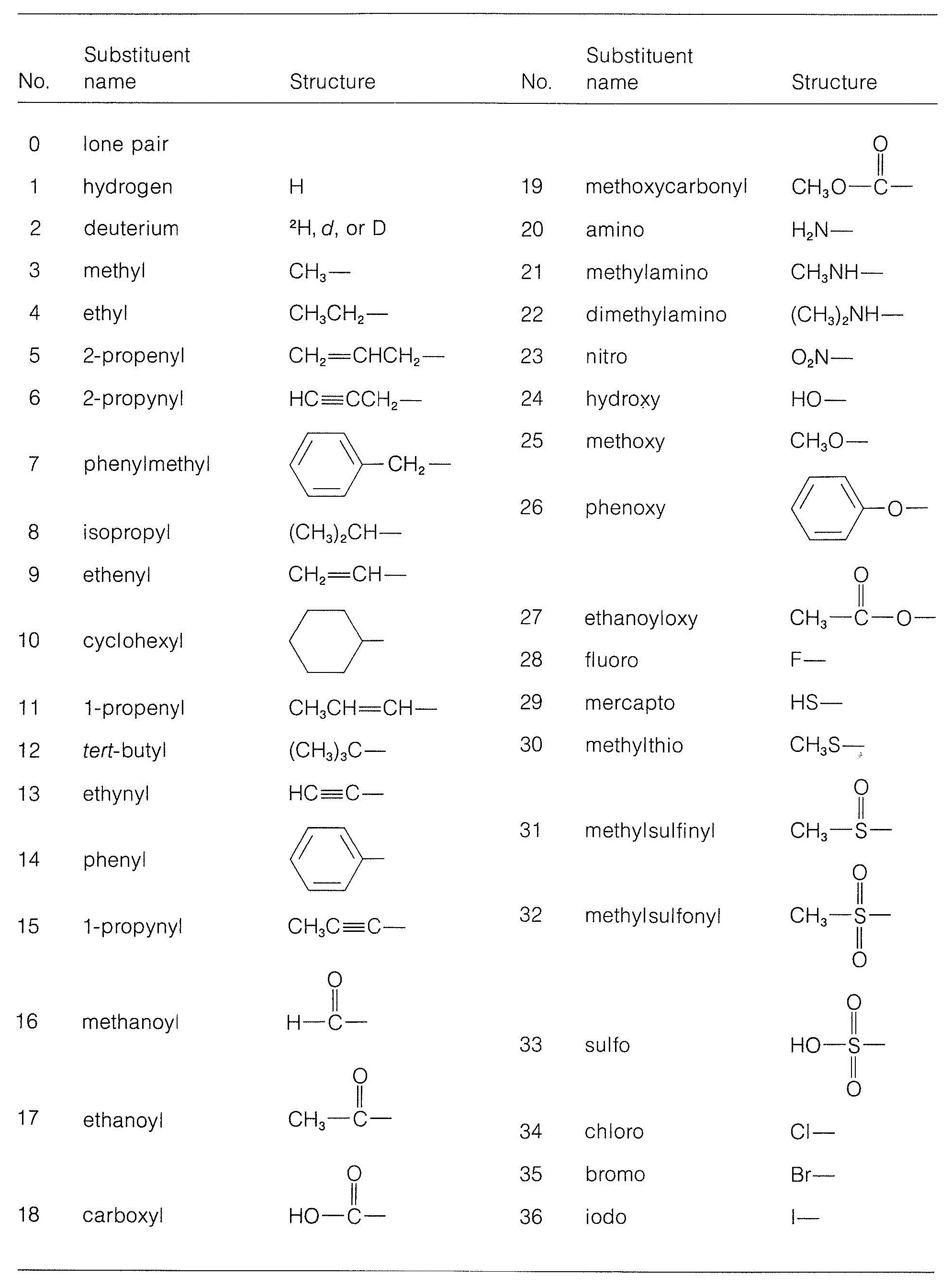
A more comprehensive list of priorities among groups is given in Table 19-1. It will be a good exercise to go through this list and work out how the priorities are established.
Table 19-1: Some Common Groups in Order of Increasing Sequence-Rule Preference
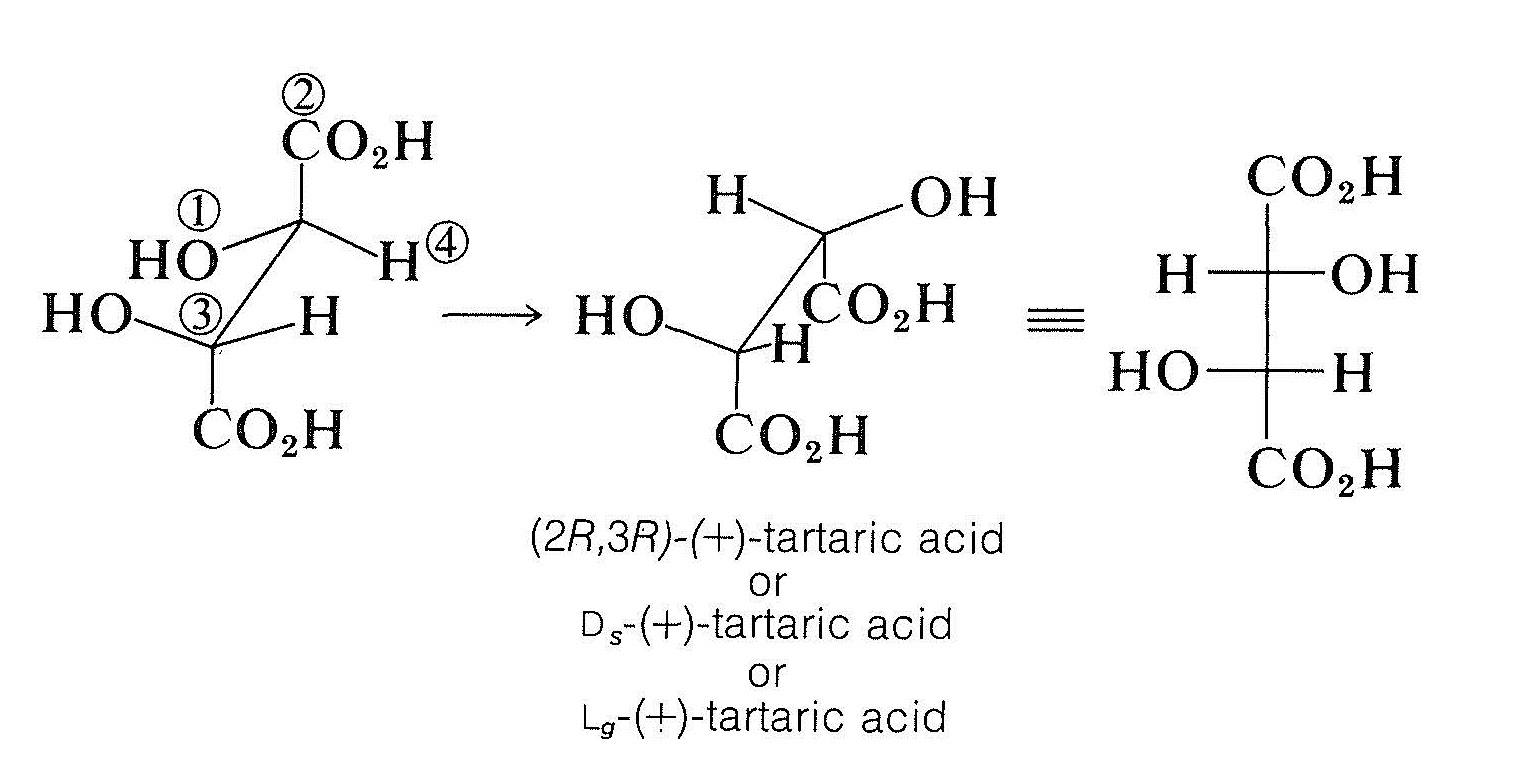
If more than one chiral center is present, the configuration at each is specified by the symbol \(R\) or \(S\) together with the number of the chiral atom. Thus the configuration of \(\left( + \right)\)-tartaric acid is known to be that designated in the name \(\left( 2R,3R \right)\)-\(\left( + \right)\)-tartaric acid:
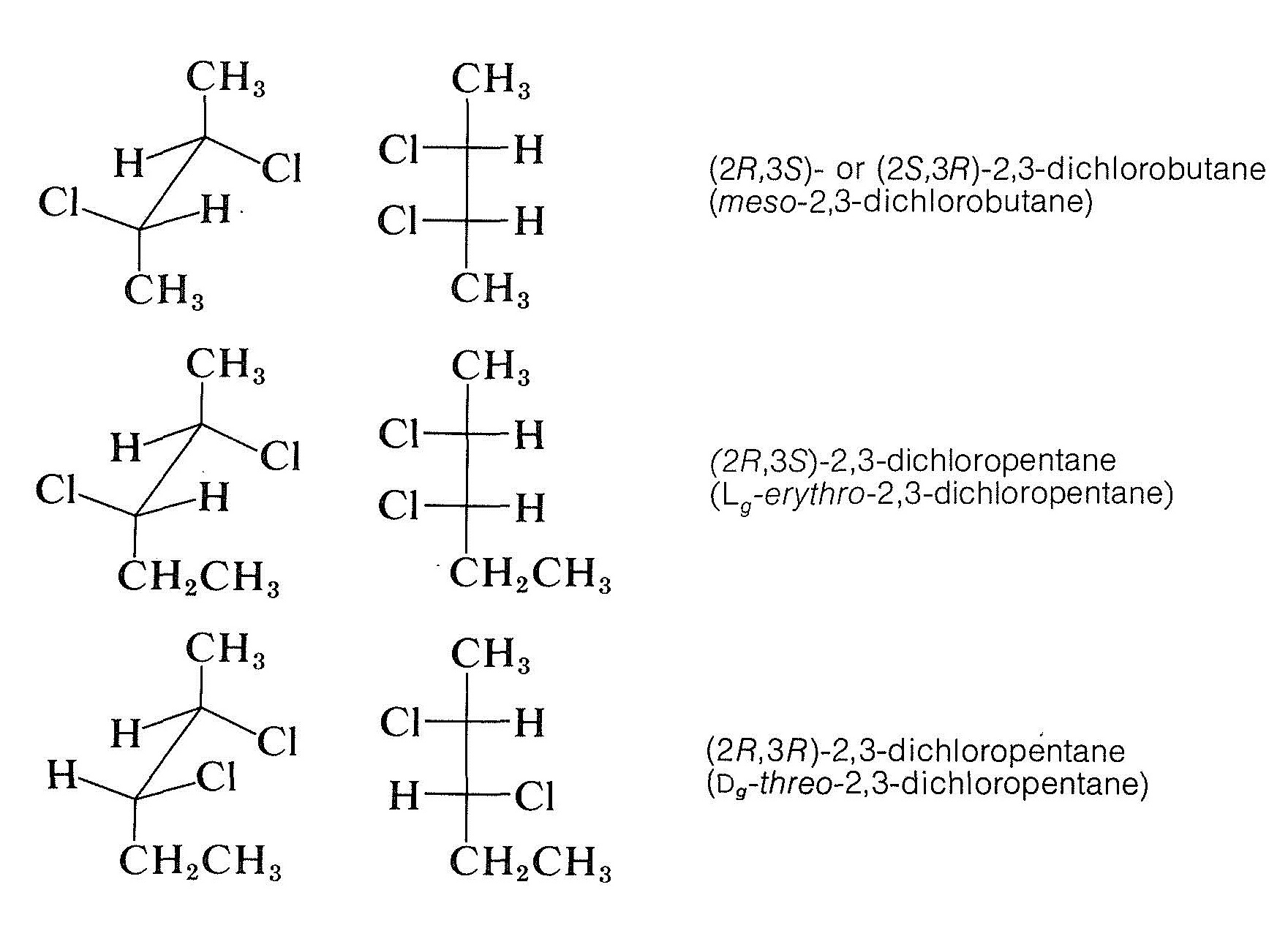
The \(R\),\(S\) system is quite general and has many advantages (and a few disadvantages) compared with the \(D\),\(L\) notation for simple molecules. For diastereomers, it provides much clearer notations than meso, erythro,\(^1\) and threo\(^1\) that have been used for many years to designate the configurations of achiral and chiral diastereomers having two chiral carbon atoms:
The \(R\),\(S\) system can be used to designate the configuration of a molecule with no chiral carbons but with a chiral center as, for example, a chiral 1,2-diene (Section 13-5A). To do this for a 1,2-diene, the molecule is best drawn in projection, looking along the \(\ce{C=C=C}\) bond with the highest-ranking group in front. The bonds in the rear will then project at \(90^\text{o}\) to the bonds of the groups in front. For a 1,2-diene, ab\(\ce{C=C=C}\)xy, where is the highest ranking group, the possible enantomeric projections are:
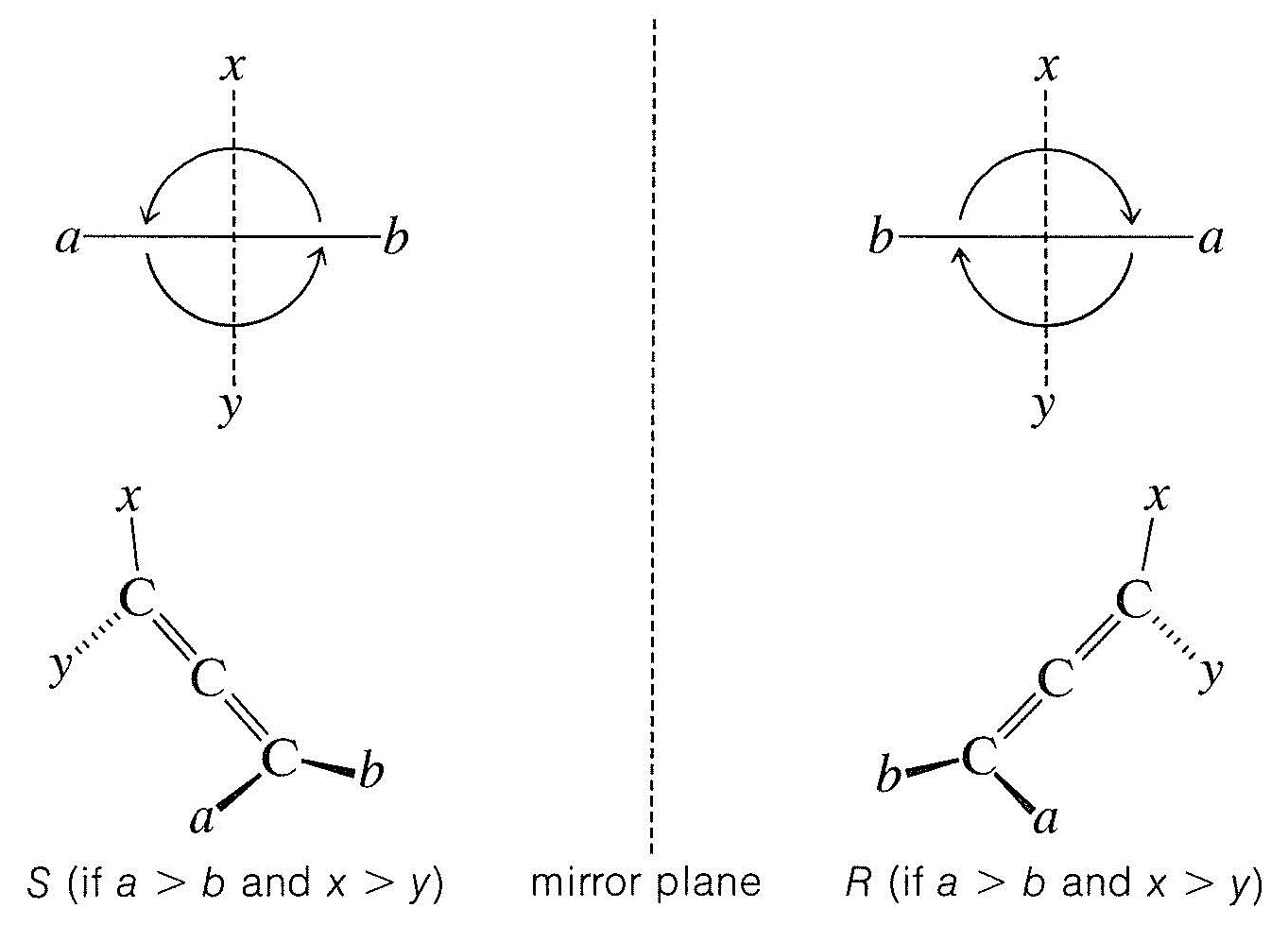
We now determine the priority of the groups and then assign the configurations \(R\) and \(S\) as shown, provided that the highest-ranking group is in front and a \(>\) b and x \(>\) y. In proceeding this way, it is important to recognize that no matter what the priority is of the group b based on atomic number, b always outranks a rear group so that the priority sequence is a \(\rightarrow\) b \(\rightarrow\) x with \(R\) clockwise and \(S\) counterclockwise.
\(^1\)The prefixes erythro and threo are used for configurations of compounds with two differently substituted chiral carbons having similar groups on each carbon. If in the Fischer projection formula the similar groups are on the same side, the configuration is erythro. If the similar groups are on opposite sides, the configuration is threo.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


