15.8: Polyhydric Alcohols
- Page ID
- 22004
The simplest example of an alcohol with more than one hydroxyl group is methanediol or methylene glycol, \(\ce{HOCH_2OH}\). The term “glycol” indicates a diol, which is a substance with two alcoholic hydroxyl groups. Methylene glycol is reasonably stable in water solution, but attempts to isolate it lead only to its dehydration product, methanal (formaldehyde):
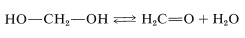
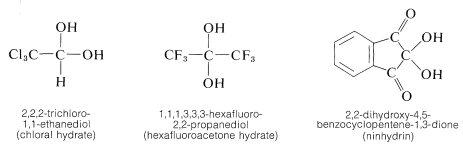
This behavior is rather typical of gem-diols (gem \(=\) geminal, that is, with both hydroxyl groups on the same carbon atom). The few gem-diols of this kind that can be isolated are those that carry strongly electron-attracting substituents such as the following:
Polyhydric alcohols in which the hydroxyl groups are situated on different carbons are relatively stable, and, as we might expect for substances with multiple polar groups, they have high boiling points and considerable water solubility, but low solubility in nonpolar solvents:
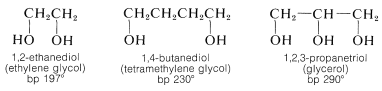
1,2-Diols are prepared from alkenes by oxidation with reagents such as osmium tetroxide, potassium permanganate, or hydrogen peroxide (Section 11-7C). However, ethylene glycol is made on a commercial scale from oxacyclopropane, which in turn is made by air oxidation of ethene at high temperatures over a silver oxide catalyst (Section 11-7D).
Ethylene glycol has important commercial uses. It is an excellent permanent antifreeze for automotive cooling systems because it is miscible with water in all proportions and a \(50\%\) solution freezes at \(-34^\text{o}\) \(\left( -29^\text{o} \text{F} \right)\). It also is used as a solvent and as an intermediate in the production of polymers (polyesters) and other products (Chapter 29).
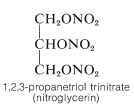
The trihydric alcohol, 1,2,3-propanetriol (glycerol), is a nontoxic, water-soluble, viscous, hygroscopic liquid that is used widely as a humectant (moistening agent). It is an important component of many food, cosmetic, and pharmaceutical preparations. At one time, glycerol was obtained on a commercial scale only as a by-product of soap manufacture through hydrolysis of fats, which are glyceryl esters of long-chain alkanoic acids. The major present source is by synthesis from propene (Section 14-3A). The trinitrate ester of glycerol (nitroglycerin) is an important but shock-sensitive explosive:
Dynamite is a much safer and more controllable explosive, and is made by absorbing nitroglycerin in porous material such as sawdust or diatomaceous earth. Dynamite has largely been replaced by cheaper explosives containing ammonium nitrate as the principal ingredient.
Glycerol, as a constituent of fats and lipids, plays an important role in animal metabolism.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


