10.7: Nucleophilic Addition Reactions
- Page ID
- 22243
When a stepwise ionic addition reaction involves nucleophilic attack at carbon as a first step, it is described as a nucleophilic addition. Reactions of this type often are catalyzed by bases, which generate the required nucleophile. For example, consider the addition of some weakly acidic reagent \(\ce{HX}\) to an alkene. In the presence of a strong base \(\left( ^\ominus \ce{OH} \right)\), \(\ce{HX}\) could give up its proton to form the conjugate base \(\ce{X}^\ominus\), which is expected to be a much better nucleophile than \(\ce{HX}\):
\[\ce{H}:\ce{X} + ^\ominus \ce{OH} \rightleftharpoons \ce{H_2O} + :\ce{X}^\ominus\]
What can follow with an alkene is an ionic chain reaction with the following two propagating steps. First, the nucleophile attacks at carbon to form a carbon anion (carbanion) intermediate (Equation 10-8). Second, electrophilic transfer of a proton from \(\ce{HX}\) to the carbanion forms the adduct and regenerates the nucleophile (Equation 10-9). The overall reaction is the addition of \(\ce{HX}\) to the double bond:
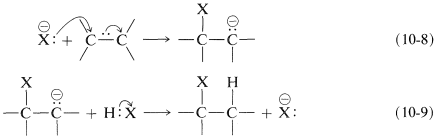
Net reaction: 
The \(\ce{HX}\) reagent can be water, an alcohol \(\left( \ce{ROH} \right)\), a thiol \(\left( \ce{RSH} \right)\), an amine \(\left( \ce{RNH_2} \right)\), or hydrogen cyanide \(\left( \ce{HCN} \right)\) or other carbon acids (i.e., compounds with acidic \(\ce{C-H}\) bonds). However, nucleophilic addition of these reagents to simple alkenes rarely is encountered. To have nucleophilic addition the double bond must be substituted with strongly electron-withdrawing groups such as carbonyl-containing groups, \(\ce{NO_2}\), \(\ce{C \equiv N}\), or positively charged ammonium or sulfonium groups. However, alkynes generally are more reactive towards nucleophiles than they are toward electrophiles. For example, with a base catalyst, 2-hexen-4-yne adds methanol across the triple bond, leaving the double bond untouched:
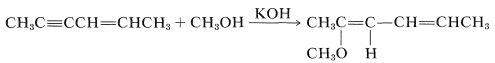
(Nonetheless, the double bond seems to be necessary because a corresponding addition is not observed for 2-butyne, \(\ce{CH_3C \equiv CCH_3}\).)
Many nucleophilic addition reactions have considerable synthetic value, particularly those involving addition of carbon acids, such as \(\ce{HCN}\), because they provide ways of forming carbon-carbon bonds. More of their utility will be discussed in Chapters 14, 17, and 18.
References
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


