5.7: Some Examples of the Importance of Stereoisomerism to Biology. Biological Stereospeciflcity
- Page ID
- 22181
Symmetrical reagents do not differentiate between the members of a pair of enantiomers for the same reason that an ordinary sock fits equally well on a right foot as on a left foot. However, asymmetric or chiral reagents can differentiate between enantiomers, especially by having at least some difference in reactivity toward them. A good analogy is the comparison between the ease of putting a left shoe on a left foot and a left shoe on a right foot. The difference may not be very pronounced for simple compounds with only one or two chiral centers, but generally the larger and more complex that chiral reagent becomes, the greater is its selectivity or power to discriminate between enantiomers and diastereomers as well. The property of being able to discriminate between diastereomers is called stereospecificity, and this is an especially important characteristic of biological systems.
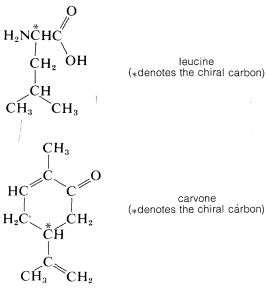
For example, our ability to taste and smell is regulated by chiral molecules in our mouths and noses that act as receptors to "sense" foreign substances. We can anticipate, then, that enantiomers may interact differently with the receptor molecules and induce different sensations. This appears to be the case. The two enantiomers of the amino acid, leucine, for example, have different tastes - one is bitter, whereas the other is sweet. Enantiomers also can smell different, as is known from the odors of the two carvones. One has the odor of caraway and the other of spearmint.
Some animals, and especially insects, rely on what amounts to a "sense-of-smell" for communication with others of their species. Substances synthesized by a particular species, and used to send messages in this way, are called pheromones. Many of these substances have rather simple molecular structures because they must be reasonably volatile and yet they are remarkably specific in the response they induce. When stereoisomerism is possible, usually only one isomer is effective. The sex attractant of the silkworm moth Bombyx mori has been identified as trans-10-cis-12-hexadecaden-1-ol, \(30\), familiarly known as "bombykol," and that of the gypsy moth is 2-methyl-cis-7-epoxy-octadecane, \(31\), or "disparlure":
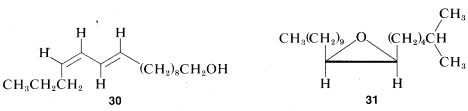
There is hope that insect sex lures can be used to disrupt the mating pattern of insects and thereby control insect population. This approach to pest control has important advantages over conventional insecticides in that the chemical lures are specific for a particular species; also they are effective in remarkably low concentrations and are relatively nontoxic. There are problems, however, not the least of which is the isolation and identification of the sex attractant that is produced by the insects only in minute quantities. Also, synergistic effects are known to operate in several insect species such that not one but several pheromones act in concert to attract the opposite sex. Two notable pests, the European corn borer and the red-banded leaf roller, both use cis-11-tetradecenyl ethanoate, \(32\), as the primary sex attractant, but the pure cis isomer is ineffective unless a small amount of trans isomer also is present. The optimum amount appears to be between \(4\%\) and \(7\%\) of the trans isomer.
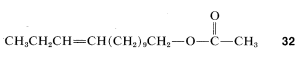
We shall discuss many other examples of biological stereospecificity in later chapters.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


