3.4: Alkenes, Cycloalkenes, and Alkadienes
- Page ID
- 22167
The open-chain hydrocarbons with one double bond have the general formula \(C_nH_{2n}\) and are called alkenes. The carbon-carbon double bond often is called an "olefinic linkage" and the alkenes designated as olefins (oil-formers). These terms arose because the gaseous lower-molecular-weight alkenes yield "oily" products on treatment with chlorine or bromine. The term "unsaturated" hydrocarbon also is used - again because these substances normally react with bromine and chlorine and are hence "unsaturated" with reference to reagents of this type (also see Section 1-1I).
According to the IUPAC system for naming alkenes, the longest continuous chain containing the double bond is given the name of the corresponding alkane with the ending -ane changed to -ene. This chain then is numbered so that the position of the first carbon of the double bond is indicated by the lowest possible number:
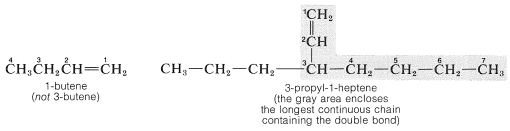
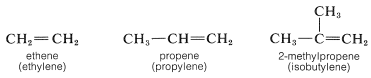
A few very common alkenes also are called "alkylenes" by appending the suffix -ene to the name of the hydrocarbon radical with the same carbon skeleton. Examples are shown below with their alkylene names in parentheses. We shall continue to use the IUPAC names whenever possible.
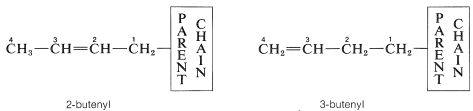
The hydrocarbon groups derived from alkenes have the suffix -enyl, as in alkenyl, and numbering of the group starts with the carbon atom attached to the main chain:
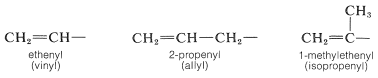
A few alkenyl groups have trivial names that commonly are used in place of systematic names. These are vinyl, allyl, and isopropenyl. And again we shall avoid using these names, except parenthetically:
Also, hydrogen atoms that are bonded directly to the unsaturated carbon atoms of a double bond often are called vinyl hydrogens, although the term alkenic hydrogens is more accurate and therefore preferable.
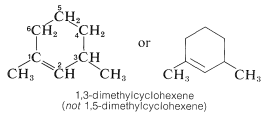
Cycloalkenes are named by the system used for the open-chain alkenes, except that numbering always is started at one of the carbons of the double bond and continued around the ring through the double bond so as to keep the index numbers as small as possible:
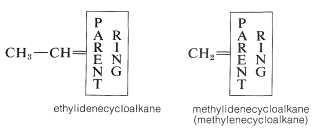
When a hydrocarbon group is double-bonded to a single carbon of a cycloalkane ring, the suffix -ylidene, as in alkylidene, is used:
Many compounds contain two or more double bonds and are known as alkadienes, alkatrienes, alkatetraenes, and so on, the suffix denoting the number of double bonds. The location of each double bond is specified by appropriate numbers, as illustrated below:
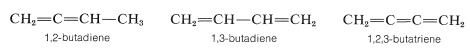
A further classification is used for the relationships of the double bonds to each other. Thus 1,2-alkadienes and similar substances are said to have cumulated double bonds:

1,3-Alkadienes and other compounds with alternating double and single bonds are said to have conjugated double bonds:
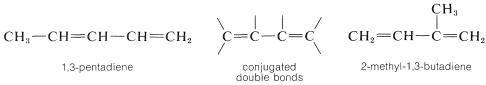
Compounds with double bonds that are neither cumulated nor conjugated are classified as having isolated double-bond systems:
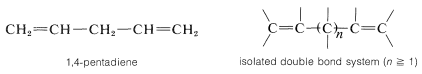
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


