1.4: Why Is Organic Chemistry Special?
- Page ID
- 22162
Let us consider some of the factors that make so much of chemistry center on a single element, carbon. One very important feature is that carbon-carbon bonds are strong, so long chains or rings of carbon atoms bonded to one another are possible. Diamond and graphite are two familiar examples, the diamond lattice being a three-dimensional network of carbon atoms, whereas graphite actually more closely resembles a planar network. The lubricating properties of graphite actually are related to its structure, which permits the planes to slide one past the other.

But carbon is not unique in forming bonds to itself because other elements such as boron, silicon, and phosphorus form strong bonds in the elementary state. The uniqueness of carbon stems more from the fact that it forms strong carbon-carbon bonds that also are strong when in combination with other elements. For example, the combination of hydrogen with carbon affords a remarkable variety of carbon hydrides, or hydrocarbons as they usually are called. In contrast, none of the other second-row elements except boron gives a very extensive system of stable hydrides, and most of the boron hydrides are much more reactive than hydrocarbons, especially to water and air.
Carbon forms bonds not only with itself and with hydrogen but also with many other elements, including strongly electron-attracting elements such as fluorine and strongly electropositive metals such as lithium:
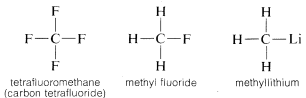
Why is carbon so versatile in its ability to bond to very different kinds of elements? The special properties of carbon can be attributed to its being a relatively small atom with four valence electrons. To form simple saltlike compounds such as sodium chloride, \(Na^\oplus Cl^\ominus\), carbon would have to either lose the four valence electrons to an element such as fluorine and be converted to a quadripositive ion, \(C^{4 \oplus}\), or acquire four electrons from an element such as lithium and form a quadrinegative ion, \(C^{4 \ominus}\). Gain of four electrons would be energetically very unfavorable because of mutual repulsion between the electrons.
Customarily, carbon completes its valence-shell octet by sharing electrons with other atoms. In compounds with shared electron bonds (or covalent bonds) such as methane, ethane, or tetrafluoromethane, each of the bonded atoms including carbon has its valence shell filled, as shown in the following electron-pair or Lewis\(^6\) structures:
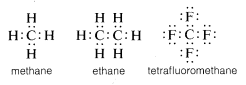
In this way, repulsions between electrons associated with completion of the valence shell of carbon are compensated by the electron-attracting powers of the positively charged nuclei of the atoms to which the carbon is bonded.
However, the electrons of a covalent bond are not necessarily shared equally by the bonded atoms, especially when the affinities of the atoms for electrons are very different. Thus, carbon-fluorine and carbon-lithium bonds, although they are not ionic, are polarized such that the electrons are associated more with the atom of higher electron affinity. This is usually the atom with the higher effective nuclear charge.
\(\overset{\delta \oplus}{C} \: \: \: \: \: : \overset{\delta \ominus}{F} \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \overset{\delta \ominus}{C:} \: \: \: \: \: \overset{\delta \oplus}{Li} \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \scriptsize{\left( \delta \oplus, \: \delta \ominus \: \text{denote partial ionic bonds} \right)}\)
We see then a gradation from purely ionic to purely covalent bonding in different molecules, and this is manifest in their chemical and physical properties. Consider, for instance, the hydrides of the elements in the second horizontal row of the periodic table. Their melting and boiling points,\(^7\) where known, are given below.
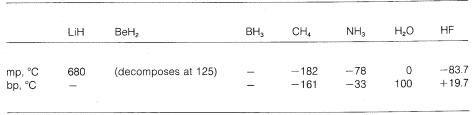
Lithium hydride can be regarded as a saltlike ionic compound, \(\overset{\oplus}{Li} \: \: \: : \overset{\ominus}{H}\). Electrostatic attractions between oppositely charged ions in the crystal lattice are strong, thereby causing lithium hydride to be a high-melting, nonvolatile solid like sodium chloride, lithium fluoride, and so on.
Methane, \(CH_4\), is at the other extreme. It boils at \(-161^\text{o}\), which is about \(800^\text{o}\) lower even than the melting point of lithium hydride. Because carbon and hydrogen have about the same electron-attracting power, \(C-H\) bonds have little ionic character, and methane may be characterized as a nonpolar substance. As a result, there is relatively little electrostatic attraction between methane molecules and this allows them to "escape" more easily from each other as gaseous molecules - hence the low boiling point.
Hydrogen fluoride has a boiling point some \(200^\text{o}\) higher than that of methane. The bonding electron pair of \(HF\) is drawn more toward fluorine than to hydrogen so the bond may be formulated as \(\overset{\delta \oplus}{H}\) ---- \(\overset{\delta \ominus}{F}\). In liquid hydrogen fluoride, the molecules tend to aggregate through what is called hydrogen bonding in chains and rings arranged so the positive hydrogen on one molecule attracts a negative fluorine on the next:
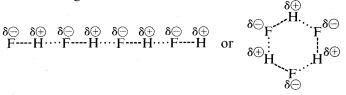
When liquid hydrogen fluoride is vaporized, the temperature must be raised sufficiently to overcome these intermolecular electrostatic attractions; hence the boiling point is high compared to liquid methane. Hydrogen fluoride is best characterized as a polar, but not ionic, substance. Although the \(O-H\) and \(N-H\) bonds of water and ammonia have somewhat less ionic character than the \(H-F\) bonds of hydrogen fluoride, these substances also are relatively polar in nature and also associate through hydrogen bonding in the same way as does hydrogen fluoride.
The chemical properties of lithium hydride, methane, and hydrogen fluoride are in accord with the above formulations. Thus, when the bond to the hydrogen is broken, we might expect it to break in the sense \(\begin{array}{c:c} Li^\oplus & :H^\ominus \end{array}\) for lithium hydride, and \(\begin{array}{c:c} \overset{\delta \oplus}{H} & : \underset{\cdot \cdot}{\ddot{F}}:^{\delta \ominus} \end{array}\) for hydrogen fluoride so that the electron pair goes with the atom of highest electron affinity. This is indeed the case as the following reaction indicates:
\(\begin{array}{c:c} Li^\oplus & :H^\ominus \end{array} + \begin{array}{c:c} H & : \underset{\cdot \cdot}{\ddot{F}} : \end{array} \longrightarrow Li^\oplus : \underset{\cdot \cdot}{\ddot{F}}:^\oplus + \: H : H\)
Methane, with its relatively nonpolar bonds, is inert to almost all reagents that could remove hydrogen as \(H^\oplus\) or \(H:^\ominus\) except under anything but extreme conditions. As would be expected, methyl cations \(CH_3^\oplus\) and methyl anions \(CH_3:^\ominus\) are very difficult to generate and are extremely reactive. For this reason, the following reactions are not observed:

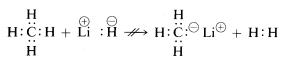
From the foregoing you may anticipate that the chemistry of carbon compounds will be largely the chemistry of covalent compounds and will not at all resemble the chemistry of inorganic salts such as sodium chloride. You also may anticipate that the major differences in chemical and physical properties of organic compounds will arise from the nature of the other elements bonded to carbon. Thus methane is not expected to, nor does it have, the same chemistry as other one-carbon compounds such as methyllithium, \(CH_3Li\), or methyl fluoride, \(CH_3F\).
\(^6\)G. N. Lewis (1876-1946), the renowned U.S. chemist, was the first to grasp the significance of the electron-pair in molecular structure. He laid the foundation for modern theory of structure and bonding in his treatise on Valence and the Structure of Atoms and Molecules (1923).
\(^7\)Throughout this text all temperatures not otherwise designated should be understood to be in \(^\text{o}C\); absolute temperatures will be shown as \(^\text{o}K\).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


