2.1: Atomic Theory and the Structure of Atoms
- Page ID
- 86188
- State the modern atomic theory.
- Describe how atoms are constructed.
The smallest piece of an element that maintains the identity of that element is called an atom. Individual atoms are extremely small. It would take about fifty million atoms in a row to make a line that is 1 cm long. The period at the end of a printed sentence has several million atoms in it. Atoms are so small that it is difficult to believe that all matter is made from atoms-but it is.
The modern atomic theory, proposed about 1803 by the English chemist John Dalton, is a fundamental concept that states that all elements are composed of atoms. Previously, we defined an atom as the smallest part of an element that maintains the identity of that element. Individual atoms are extremely small; even the largest atom has an approximate diameter of only 5.4 × 10−10 m. With that size, it takes over 18 million of these atoms, lined up side by side, to equal the width of your little finger (about 1 cm).
Dalton studied the weights of various elements and compounds. He noticed that matter always combined in fixed ratios based on weight, or volume in the case of gases. Chemical compounds always contain the same proportion of elements by mass, regardless of amount, which provided further support for Proust's law of definite proportions. Dalton also observed that there could be more than one combination of two elements.
From his experiments and observations, as well as the work from peers of his time, Dalton proposed a new theory of the atom. This later became known as Dalton's atomic theory. The general tenets of this theory were as follows:
- All matter is composed of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be subdivided, created, or destroyed.
- Atoms of different elements can combine in simple whole number ratios to form chemical compounds.
- In chemical reactions, atoms are combined, separated, or rearranged.
Dalton's atomic theory has been largely accepted by the scientific community, with the exception of three changes. We know now that (1) an atom can be further subdivided, (2) all atoms of an element are not identical in mass, and (3) using nuclear fission and fusion techniques, we can create or destroy atoms by changing them into other atoms.
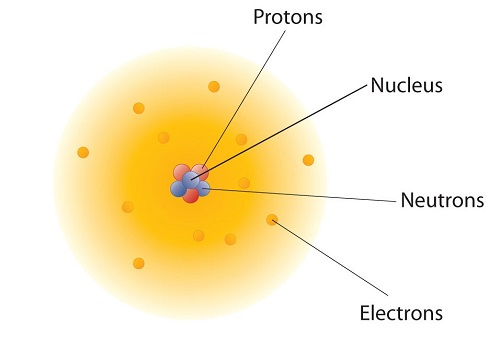
These concepts form the basis of chemistry. Although the word atom comes from a Greek word that means "indivisible," we understand now that atoms themselves are composed of smaller parts called subatomic particles. The first part to be discovered was the electron, a tiny subatomic particle with a negative charge. It is often represented as e−, with the right superscript showing the negative charge. Later, two larger particles were discovered. The proton, a subatomic particle with a positive charge. is a more massive (but still tiny) subatomic particle with a positive charge, represented as p+. The neutron is a subatomic particle with about the same mass as a proton but no charge. It is represented as either n or n0. We now know that all atoms of all elements are composed of electrons, protons, and (with one exception) neutrons. Table \(\PageIndex{1}\) summarizes the properties of these three subatomic particles.
| Name | Symbol | Mass (approx.; g) | Mass (approx.; amu) | Charge |
|---|---|---|---|---|
| Proton | p+ | 1.673 × 10−24 | 1.0073 | +1 |
| Neutron | n, n0 | 1.675 × 10−24 | 1.0087 | none |
| Electron | e− | 9.109 × 10−28 | 5.486 × 10−4 | –1 |
Atoms and subatomic particles are so small that it doesn't quite make sense to measure their masses in grams. A more useful unit to measure atomic mass is the atomic mass unit (\(\text{amu}\)), where 1 amu = 1.660539 × 10−24 g or one-twelfth of the mass of a carbon-12 atom. As you can see in the table above, the mass of 1 proton and 1 neutron are each 1 amu in this system. Carbon-12 contains six protons and six neutrons and is assigned a mass of exactly 12 amu.

How are these subatomic particles arranged in atoms? They are not arranged at random. Experiments by Ernest Rutherford in England in the 1910's pointed to a nuclear model with atoms that has the protons and neutrons in a central nucleus with the electrons in orbit about the nucleus. The relatively massive protons and neutrons are collected in the center of an atom, in a region called the nucleus of the atom (plural nuclei). The electrons are outside the nucleus and spend their time orbiting in space about the nucleus. (Figure \(\PageIndex{2}\)). Because protons and neutrons are so massive compared to electrons, Table \(\PageIndex{1}\), nearly all of the mass of an atom is contained in the nucleus.
The evidence for atoms is so great that few doubt their existence. In fact, individual atoms are now routinely observed with state-of-the art technologies. Moreover, they can even be used for making pretty images or as IBM research demonstrate in Video \(\PageIndex{1}\), control of individual atoms can be use used create animations.
Video \(\PageIndex{1}\): A Boy And His Atom - The World's Smallest Movie. A Boy and His Atom is a 2012 stop-motion animated short film released by IBM Research. The movie tells the story of a boy and a wayward atom who meet and become friends. It depicts a boy playing with an atom that takes various forms. It was made by moving carbon monoxide molecules viewed with a scanning tunneling microscope, a device that magnifies them 100 million times. These molecules were moved to create images, which were then saved as individual frames to make the film.
Key Takeaways
- Chemistry is based on the modern atomic theory, which states that all matter is composed of atoms.
- Atoms themselves are composed of protons, neutrons, and electrons.
- Each element has its own atomic number, which is equal to the number of protons in its nucleus.

