19.7: Enzyme Regulation- Allosteric Control and Feedback Inhibition
- Page ID
- 86761
- Objective 1
- Objective 2
In the previous section you learned about the different types of enzyme inhibitors and how they can be used to slow or stop enzyme activity by binding to an enzyme or enzyme-substrate complex. All of these inhibitor types, except noncompetitive inhibitors, work by binding to enzyme active sites. Noncompetitive inhibitors, however, work by binding to an enzyme at a location other than the active site, an allosteric site. Inhibitors and other molecules, called activators, that bind to enzymes at allosteric sites are considered an important part of enzyme regulation called allosteric control. In this section, we will take a look at allosteric control and feedback control, two ways in which enzyme activity is regulated differently.
Allosteric Control
Allosteric enzymes have both a binding site, for substrate binding and catalysis, and an allosteric site, for regulation of enzyme activity. When a regulator molecule binds to the allosteric site of an enzyme, usually by noncovalent interactions, a conformational change occurs in the enzyme active site, which affects substrate binding and reaction rates. Allosteric regulation of enzyme activity can be either positive, increasing reaction rates, or negative, decreasing reaction rates.
When an enzyme binds a negative regulator (or inhibitor), it will undergo a change in the active site in a way that prevents substrate binding, thereby lowering the reaction rate. As illustrated in the left-hand panel in Figure \(\PageIndex{1}\), the active site changes (becomes smaller in this case) and the substrate can no longer bind. Positive regulators (activators) bind to allosteric sites and cause conformational changes that open up an active site to promote substrate binding, allowing catalysis or increasing the reaction rate. The right panel of Figure \(\PageIndex{1}\) shows an enzyme that will only bind substrate when the active site is formed after the allosteric activator binds.
Some enzymes will have more than one allosteric site that can interact with one another, which allows for highly-controlled or finely-tuned reaction rates.

Feedback Control
Many biological processes involve the sequential action of multiple enzymes, a reaction pathway, in which the product of one reaction is the substrate for the next enzyme and so on until the final product is formed. Positive or negative regulation of these pathways often occurs by feedback control, where a product from one of the steps in the path feedback to an earlier step in the process to increase or decrease production. It may help to visualize a factory assembly line with each person responsible for one step (catalytic reaction) in making a perfect box of 12 donuts. If the last person in the line, who is responsible for putting 12 donuts in the box, falls behind, donuts will start to pile up. In order to not waste donuts or have less than full boxes at the end, it would be beneficial to signal to the other people to slow down or take a break. The process is similar in biochemical pathways: if too much product is being formed, the pathway needs to be turned off so energy and resources are not wasted.
Consider the pathway shown below in which substrate A is converted to product D through three enzymes and two intermediate products (B and C):
\(A\ \xrightarrow{Enzyme\ 1}\ B\ \xrightarrow{Enzyme\ 2}\ C\ \xrightarrow{Enzyme\ 3}\ D\)
If there is a lot of product D formed, there would be enough to bind to Enzyme 1, which would inhibit formation of products B, and subsequently product C, and D. This type of feedback control is useful to prevent waste of substrate A and any energy that is needed for the activity of Enzymes 1-3. As you will see in later chapters, there are many different types of feedback control that can both negatively and positively regulate pathways. Typically, feedback control occurs at points in pathways where it would be energetically unfavorable to proceede if the final product is not needed.
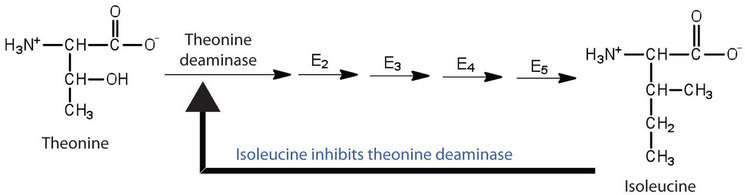
Feedback inhibition is used to regulate the synthesis of many amino acids. For example, bacteria synthesize isoleucine from threonine in a series of five enzyme-catalyzed steps. As the concentration of isoleucine increases, some of it binds as a noncompetitive inhibitor to the first enzyme of the series (threonine deaminase), thus bringing about a decrease in the amount of isoleucine being formed (Figure \(\PageIndex{2}\)).