15.4: Some Common Aldehydes and Ketones
- Page ID
- 86289
- Objective 1
- Objective 2
Formaldehyde has an irritating odor. Because of its reactivity, it is difficult to handle in the gaseous state. For many uses, it is therefore dissolved in water and sold as a 37% to 40% aqueous solution called formalin. Formaldehyde denatures proteins, rendering them insoluble in water and resistant to bacterial decay. For this reason, formalin is used in embalming solutions and in preserving biological specimens.
Aldehydes are the active components in many other familiar substances. Large quantities of formaldehyde are used to make phenol-formaldehyde resins for gluing the wood sheets in plywood and as adhesives in other building materials. Sometimes the formaldehyde escapes from the materials and causes health problems in some people. While some people seem unaffected, others experience coughing, wheezing, eye irritation, and other symptoms.
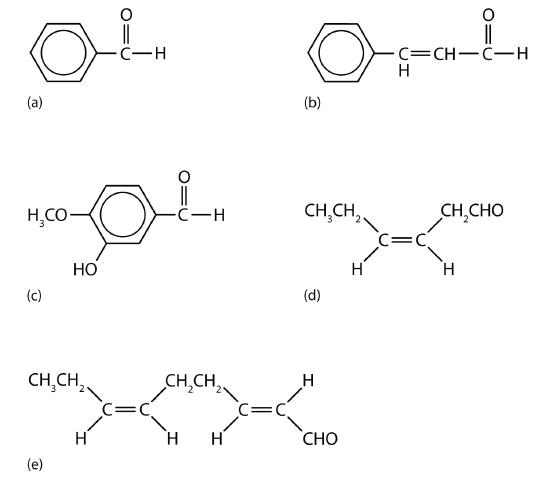
The odor of green leaves is due in part to a carbonyl compound, cis-3-hexenal, which with related compounds is used to impart a “green” herbal odor to shampoos and other products.
Acetaldehyde is an extremely volatile, colorless liquid. It is a starting material for the preparation of many other organic compounds. Acetaldehyde is formed as a metabolite in the fermentation of sugars and in the detoxification of alcohol in the liver. Aldehydes are the active components of many other familiar materials (Figure \(\PageIndex{2}\)).
Acetone is the simplest and most important ketone. Because it is miscible with water as well as with most organic solvents, its chief use is as an industrial solvent (for example, for paints and lacquers). It is also the chief ingredient in some brands of nail polish remover.
Acetone is formed in the human body as a by-product of lipid metabolism. Normally, acetone does not accumulate to an appreciable extent because it is oxidized to carbon dioxide and water. The normal concentration of acetone in the human body is less than 1 mg/100 mL of blood. In certain disease states, such as uncontrolled diabetes mellitus, the acetone concentration rises to higher levels. It is then excreted in the urine, where it is easily detected. In severe cases, its odor can be noted on the breath.
Ketones are also the active components of other familiar substances, some of which are noted in the accompanying figure.
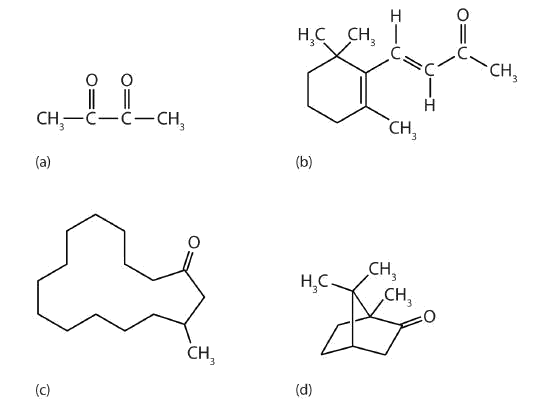
Some ketones have interesting properties: (a) Butter flavoring comes from 2,3-butanedione; (b) β-ionone is responsible for the odor of violets; (c) muscone is musk oil, an ingredient in perfumes; and (d) camphor is used in some insect repellents.
Certain steroid hormones have the ketone functional group as a part of their structure. Two examples are progesterone, a hormone secreted by the ovaries that stimulates the growth of cells in the uterine wall and prepares it for attachment of a fertilized egg, and testosterone, the main male sex hormone. These and other sex hormones affect our development and our lives in fundamental ways.

