21.7: Lewis Acids and Bases
- Page ID
- 53936
Ideas in science do not stay static. One discovery builds upon another. The concept of acids and bases has grown from the fundamental ideas of Arrhenius to Brønsted-Lowry to Lewis. Each step adds to our understanding of the surrounding world, and makes the "big picture" even bigger.
Lewis Acids and Bases
Gilbert Lewis (1875-1946) proposed a third theory of acids and bases that is even more general than either the Arrhenius or Brønsted-Lowry theories. A Lewis acid is a substance that accepts a pair of electrons to form a covalent bond. A Lewis base is a substance that donates a pair of electrons to form a covalent bond. So, a Lewis acid-base reaction is represented by the transfer of a pair of electrons from a base to an acid. A hydrogen ion, which lacks any electrons, accepts a pair of electrons. It is an acid under both the Brønsted-Lowry and Lewis definitions. Ammonia consists of a nitrogen atom as the central atom with a lone pair of electrons. The reaction between ammonia and the hydrogen ion can be depicted as shown in the figure below.

The lone pair on the nitrogen atom is transferred to the hydrogen ion, making the \(\ce{NH_3}\) a Lewis base while the \(\ce{H^+}\) is a Lewis acid.
Some reactions that do not qualify as acid-base reactions under the other definitions do so under only the Lewis definition. An example is the reaction of ammonia with boron trifluoride.
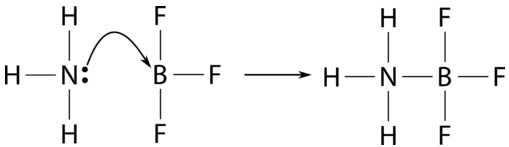
Boron trifluoride is the Lewis acid, while ammonia is again the Lewis base. As there is no hydrogen ion involved in this reaction, it qualifies as an acid-base reaction only under the Lewis definition. The table below summarizes the three acid-base theories.
| Table \(\PageIndex{1}\): Acid-Base Definitions | ||
|---|---|---|
| Type | Acid | Base |
| Arrhenius | \(\ce{H^+}\) ions in solution | \(\ce{OH^-}\) ions in solution |
| Brønsted-Lowry | \(\ce{H^+}\) donor | \(\ce{H^+}\) acceptor |
| Lewis | electron-pair acceptor | electron-pair donor |
Summary
- A Lewis acid is a substance that accepts a pair of electrons to form a covalent bond.
- A Lewis base is a substance that donates a pair of electrons to form a covalent bond.
- Examples of Lewis acids and bases are given.

