16.17: Molecular and Ionic Equations
- Page ID
- 53865
One of the unfortunate byproducts of industrialized society is acid rain. Sulfur dioxide from burning coal and nitrogen oxides from vehicle emissions both form acids. When these acids react with limestone (calcium carbonate), reactions occur that dissolve the limestone and release water and carbon dioxide. Over a period of time, serious damage is caused to the limestone structure.
Molecular and Ionic Equations
When ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal lattice, resulting in the hydrated ions being evenly distributed through the water. This process is called dissociation and is the reason that all ionic compounds are strong electrolytes. When two different ionic compounds that have been dissolved in water are mixed, a chemical reaction may occur between certain pairs of the hydrated ions. Consider the double-replacement reaction that occurs when a solution of sodium chloride is mixed with a solution of silver nitrate:
\[\ce{NaCl} \left( aq \right) + \ce{AgNO_3} \left( aq \right) \rightarrow \ce{NaNO_3} \left( aq \right) + \ce{AgCl} \left( s \right)\nonumber \]
The driving force behind this reaction is the formation of the silver chloride precipitate (see figure below).
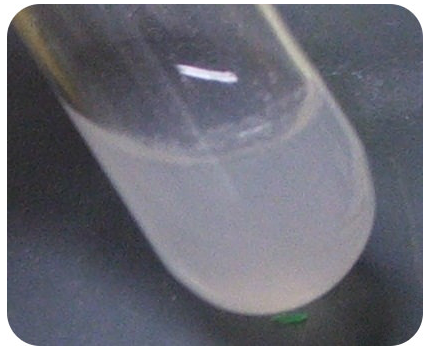
This is called a molecular equation. A molecular equation is an equation in which the formulas of the compounds are written as though all substances exist as molecules. However, there is a better way to show what is happening in this reaction. All of the aqueous compounds should be written as ions, because they are present in the water as separated ions—a result of their dissociation.
\[\ce{Na^+} \left( aq \right) + \ce{Cl^-} \left( aq \right) + \ce{Ag^+} \left( aq \right) + \ce{NO_3^-} \left( aq \right) \rightarrow \ce{Na^+} \left( aq \right) + \ce{NO_3^-} \left( aq \right) + \ce{AgCl} \left( s \right)\nonumber \]
This equation is called an ionic equation, an equation in which dissolved ionic compounds are shown as free ions.
Some other double-replacement reactions do not produce a precipitate as one of the products. The production of a gas and/or a molecular compound such as water may also drive the reaction. For example, consider the reaction of a solution of sodium carbonate with a solution of hydrochloric acid \(\left( \ce{HCl} \right)\). The products of the reaction are aqueous sodium chloride, carbon dioxide, and water. The balanced molecular equation is:
\[\ce{Na_2CO_3} \left( aq \right) + 2 \ce{HCl} \left( aq \right) \rightarrow 2 \ce{NaCl} \left( aq \right) + \ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right)\nonumber \]
The ionic equation is:
\[2 \ce{Na^+} \left( aq \right) + \ce{CO_3^{2-}} \left( aq \right) + 2 \ce{H^+} \left( aq \right) + 2 \ce{Cl^-} \left( aq \right) \rightarrow 2 \ce{Na^+} \left( aq \right) + 2 \ce{Cl^-} \left( aq \right) + \ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right)\nonumber \]
A single-replacement reaction is one in which an element replaces another element in a compound. An element is in either the solid, liquid, or gas state and is not an ion. The example below shows the reaction of solid magnesium metal with aqueous silver nitrate to form aqueous magnesium nitrate and silver metal.
Balanced molecular equation:
\[\ce{Mg} \left( s \right) + 2 \ce{AgNO_3} \left( aq \right) \rightarrow \ce{Mg(NO_3)_2} \left( aq \right) + 2 \ce{Ag} \left( s \right)\nonumber \]
Ionic equation:
\[\ce{Mg} \left( s \right) + 2 \ce{Ag^+} \left( aq \right) + 2 \ce{NO_3^-} \left( aq \right) \rightarrow \ce{Mg^{2+}} \left( aq \right) + 2 \ce{NO_3^-} \left( aq \right) + \ce{Ag} \left( s \right)\nonumber \]
This type of single-replacement reaction is called a metal replacement. Other common categories of single-replacement reactions are: hydrogen replacement and halogen replacement.
Summary
- A molecular equation is an equation in which the formulas of the compounds are written as though all substances exist as molecules.
- An ionic equation is one in which dissolved ionic compounds are shown as free ions.
- Examples of molecular and ionic equations are shown.

