10.8: Mole Road Map
- Page ID
- 53773
How do I get from here to there?
If I want to visit the town of Manteo, North Carolina, out on the coast, I will need a map of how to get there. I may have a printed map or I may download directions from the internet, but I need something to get me going in the right direction. Chemistry road maps serve the same purpose. How do I handle a certain type of calculation? There is a process and a set of directions to help.
Mole Road Map
Previously, we saw how the conversions between mass and number of particles required two steps, with moles as the intermediate. This concept can now be extended to also include gas volume at STP. The resulting diagram is referred to as a mole road map (see figure below).
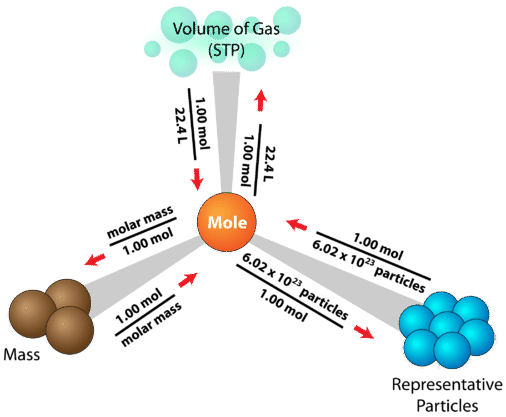
The mole is at the center of any calculation involving amount of a substance. The sample problem below is one of many different problems that can be solved using the mole road map.
Example \(\PageIndex{1}\): Mole Road Map
What is the volume of \(79.3 \: \text{g}\) of neon gas at STP?
Solution
Step 1: List the known quantities and plan the problem.
Known
- \(\ce{Ne} = 20.18 \: \text{g/mol}\)
- \(1 \: \text{mol} = 22.4 \: \text{L}\)
Unknown
- volume = ? L
The conversion factors will be grams \(\rightarrow\) moles \(\rightarrow\) gas volume.
Step 2: Calculate.
\[79.3 \: \text{g} \: \ce{Ne} \times \frac{1 \: \text{mol} \: \ce{Ne}}{20.18 \: \text{g} \: \ce{Ne}} \times \frac{22.4 \: \text{L} \: \ce{Ne}}{1 \: \text{mol} \: \ce{Ne}} = 88.0 \: \text{L} \: \ce{Ne}\nonumber \]
Step 3: Think about your result.
The given mass of neon is equal to about 4 moles, resulting in a volume that is about 4 times larger than molar volume.
Summary
- An overall process is given for calculations involving moles, grams, and gas volume.
Review
- In the problem above, what is the formula weight of neon?
- What value is at the center of all the calculations?
- If we had 79.3 grams of Xe, would we expect a volume that is greater than or less than that obtained with neon?

