9.9: Covalent Bonding in Polyatomic Ions
- Page ID
- 53749
How do we extend basic principles?
The United States Supreme Court has the unenviable task of determining what the law dictates. This responsibility can be a major challenge when there is no clear principle involved, or when they face a situation not previously encountered. Chemistry faces the same challenge in extending basic concepts to fit a new situation. Drawing of Lewis structures for polyatomic ions uses the same approach, but tweaks the process a little to fit a somewhat different set of circumstances.
Polyatomic Ions
Recall that a polyatomic ion is a group of atoms that are covalently bonded together, and which carry an overall electrical charge. The ammonium ion, \(\ce{NH_4^+}\), is formed when a hydrogen ion \(\left( \ce{H^+} \right)\) attaches to the lone pair of an ammonia \(\left( \ce{NH_3} \right)\) molecule in a coordinate covalent bond.
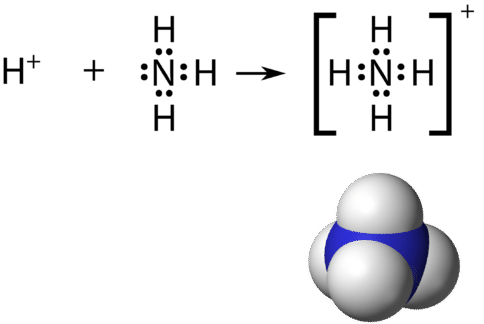
When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion:
\(1 \: \ce{N}\) atom \(= 5\) valence electrons
\(4 \: \ce{H}\) atoms \(= 4 \times 1 = 4\) valence electrons
subtract 1 electron for the \(1+\)charge of the ion
total of 8 valence electrons in the ion
It is customary to put the Lewis structure of a polyatomic ion into a large set of brackets, with the charge of the ion as a superscript outside of the brackets.
Example \(\PageIndex{1}\)
Draw the Lewis electron dot structure for the sulfate ion.
Solution
Step 1: List the known quantities and plan the problem.
Known
Molecular formula of sulfate ion: \( \ce{SO_4^{2-}}\)
\(1 \: \ce{S}\) atom \(= 6\) valence electrons
\(4 \: \ce{O}\) atoms \(= 4 \times 6 = 24\) valence electrons
add 2 electrons for the \(2-\) charge of the ion
= total of 32 valence electrons
The less electronegative sulfur atom is the central atom in the structure. Place the oxygen atoms around the sulfur atom, each with a single covalent bond. Distribute lone pairs to each oxygen atom in order to satisfy the octet rule. Count the total number of atoms. If there are too many electrons in the structure, make multiple bonds between the \(\ce{S}\) and \(\ce{O}\).
Step 2: Solve.
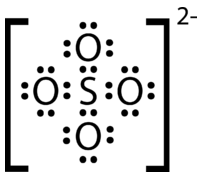
Step 3: Think about your result.
The Lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms. This yields the expected total of 32 electrons. Since the sulfur atom started with six valence electrons, two of the \(\ce{S-O}\) bonds are coordinate covalent.
Summary
- Lewis structures for polyatomic ions follow the same rules as those for other covalent compounds.
Review
- What are two characteristics of polyatomic ions?
- Which atom becomes the central atom in the structure?
- Where is the charge on an ion placed in a lewis dot diagram?

