22.4: Molecular Redox Reactions
- Page ID
- 53958
Acetone is a versatile chemical used both in manufacture of plastics and as a solvent. It is a major constituent of such products as nail polish remover, paints, and cleaning fluids. The manufacture of acetone involves formation of an intermediate peroxide compound by oxidation, followed by formation of the final product.
Molecular Redox Reactions
The electron loss and gain is easy to see in a reaction in which ions are formed. However, in many reactions, no such electron transfer occurs. In a molecular compound, electrons are shared between atoms in a type of bond called a covalent bond. Yet, it is still common for reactions involving molecular compounds to be classified as redox reactions.
When hydrogen gas is reacted with oxygen gas, water is formed as the product.
\[2 \ce{H_2} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{H_2O} \left( l \right)\nonumber \]
In the individual hydrogen molecules, a pair of bonding electrons is shared equally between the hydrogen atoms (a nonpolar covalent bond). Likewise, the bonding electrons in the oxygen molecule are also shared equally between the two oxygen atoms. However, when the atoms are rearranged to form the water molecule, the electron sharing is no longer equal. In each hydrogen-oxygen bond in the water molecule, the bonding electrons are more attracted to the oxygen atom than they are to the hydrogen atom. We know this because oxygen has a higher electronegativity than hydrogen.
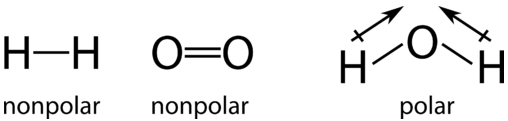
In the course of this reaction, electrons are shifted away from each hydrogen atom and towards the oxygen atom. The hydrogen is oxidized because it undergoes a partial loss of electrons. Even though the loss is not complete enough to form ions, the hydrogen atoms in water have less electron density near them than they did in the \(\ce{H_2}\) molecule. The oxygen is reduced because it undergoes a partial gain of electrons. The oxygen atom in water has greater electron density here than it did in the \(\ce{O_2}\) molecule.
Another approach to this type of problem is to go back to our earlier definitions of oxidation being the gain of oxygen or loss of hydrogen, and reduction being the gain of hydrogen or loss of oxygen. This makes the decision about redox reactions much easier. The hydrogen is oxidized because it added oxygen to form water. Conversely, the oxygen is reduced because it added hydrogen to form water.

