14.3: Boyle's Law
- Page ID
- 53822
Each day, hundreds of weather balloons are launched. Made of a synthetic rubber and carrying a box of instruments, each helium-filled balloon rises up into the sky. As a balloon gains altitude, the atmospheric pressure becomes less and the balloon expands. At some point the balloon bursts due to the expansion; the instruments drop (aided by a parachute) to be retrieved and studied for information about the weather.
Boyle's Law
Robert Boyle (1627-1691), an English chemist, is widely considered to be one of the founders of the modern experimental science of chemistry. He discovered that doubling the pressure of an enclosed sample of gas, while keeping its temperature constant, caused the volume of the gas to be reduced by half. Boyle's law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant. An inverse relationship is described in this way. As one variable increases in value, the other variable decreases.
Physically, what is happening? The gas molecules are moving and are a certain distance apart from one another. An increase in pressure pushes the molecules closer together, reducing the volume. If the pressure is decreased, the gases are free to move about in a larger volume.

Mathematically, Boyle's law can be expressed by the equation:
\[P \times V = k\nonumber \]
The \(k\) is a constant for a given sample of gas and depends only on the mass of the gas and the temperature. The table below shows pressure and volume data for a set amount of gas at a constant temperature. The third column represents the value of the constant \(\left( k \right)\) for this data and is always equal to the pressure multiplied by the volume. As one of the variables changes, the other changes in such a way that the product of \(P \times V\) always remains the same. In this particular case, that constant is \(500 \: \text{atm} \cdot \text{mL}\).
| Pressure \(\left( \text{atm} \right)\) | Volume \(\left( \text{mL} \right)\) | \(P \times V = k\) \(\left( \text{atm} \cdot \text{mL} \right)\) |
|---|---|---|
| 0.5 | 1000 | 500 |
| 0.625 | 800 | 500 |
| 1.0 | 500 | 500 |
| 2.0 | 250 | 500 |
| 5.0 | 100 | 500 |
| 8.0 | 62.5 | 500 |
| 10.0 | 50 | 500 |
A graph of the data in the table further illustrates the inverse relationship nature of Boyle's Law (see figure below). Volume is plotted on the \(x\)-axis, with the corresponding pressure on the \(y\)-axis.
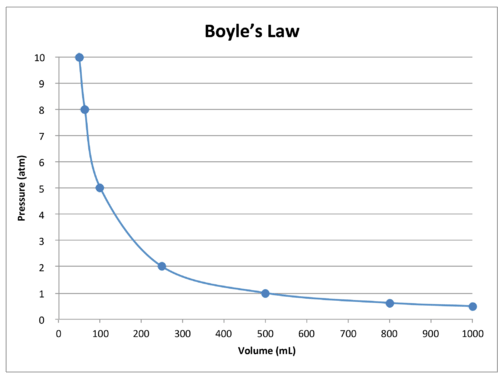
Boyle's Law can be used to compare changing conditions for a gas. We use \(P_1\) and \(V_1\) to stand for the initial pressure and initial volume of a gas. After a change has been made, \(P_2\) and \(V_2\) stand for the final pressure and volume. The mathematical relationship of Boyle's Law becomes:
\[P_1 \times V_1 = P_2 \times V_2\nonumber \]
This equation can be used to calculate any one of the four quantities if the other three are known.
Example \(\PageIndex{1}\)
A sample of oxygen gas has a volume of \(425 \: \text{mL}\) when the pressure is equal to \(387 \: \text{kPa}\). The gas is allowed to expand into a \(1.75 \: \text{L}\) container. Calculate the new pressure of the gas.
Solution
Step 1: List the known quantities and plan the problem.
Known
- \(P_1 = 387 \: \text{kPa}\)
- \(V_1 = 425 \: \text{mL}\)
- \(V_2 = 1.75 \: \text{L} = 1750 \: \text{mL}\)
Unknown
Use Boyle's Law to solve for the unknown pressure \(\left( P_2 \right)\). It is important that the two volumes (\(V_1\) and \(V_2\)) are expressed in the same units, so \(V_2\) has been converted to \(\text{mL}\).
Step 2: Solve.
First, rearrange the equation algebraically to solve for \(P_2\).
\[P_2 = \frac{P_1 \times V_1}{V_2}\nonumber \]
Now substitute the known quantities into the equation and solve.
\[P_2 = \frac{387 \: \text{kPa} \times 425 \: \text{mL}}{1750 \: \text{mL}} = 94.0 \: \text{kPa}\nonumber \]
Step 3: Think about your result.
The volume has increased to slightly over 4 times its original value and so the pressure is decreased by about one fourth. The pressure is in \(\text{kPa}\) and the value has three significant figures. Note that any pressure or volume units can be used as long as they are consistent throughout the problem.

