Group 14: General Properties and Reactions
- Page ID
- 31725
Carbon is one of the most common elements on earth, and greatly influences everyday life. Common molecules containing carbon include carbon dioxide (CO2) and methane (CH4). Many scientists in a variety of fields study of carbon: biologists investigating the origins of life; oceanographers measuring the acidification of the oceans; and engineers developing diamond film tools. This article details the periodic properties of the carbon family and briefly discusses of the individual properties of carbon, silicon, germanium, tin, lead, and flerovium.
Introduction
The carbon family, Group 14 in the p-block, contains carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). Each of these elements has only two electrons in its outermost p orbital: each has the electron configuration ns2np2. The Group 14 elements tend to adopt oxidation states of +4 and, for the heavier elements, +2 due to the inert pair effect.
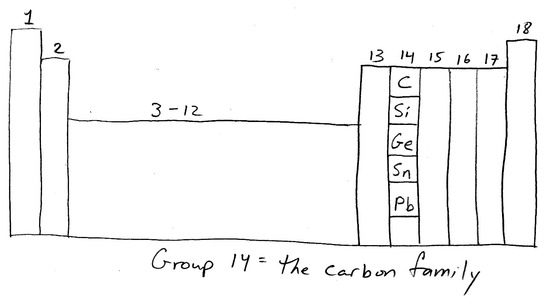
Members of this group conform well to general periodic trends. The atomic radii increase down the group, and ionization energies decrease. Metallic properties increase down the group. Carbon is a non-metal, silicon and germanium are metalloids, and tin and lead are poor metals (they conduct heat and electricity less effectively than other metals such as copper).
Despite their adherence to periodic trends, the properties of the carbon family vary greatly. For example, carbon is a non-metal and behaves as such, whereas tin and lead behave entirely as metals. In their elemental solid states, the Group 14 metalloids silicon and germanium act as electrical semiconductors, although silicon is largely non-metallic; their electrical conductivity can be affected in various degrees by doping, or adding of Group 13 or Group 15 elements in varying concentrations to the Group 14 solid matrix. These semiconductor properties have wide application for circuitry components in the electronics industry, such as diodes, transistors, and integrated circuit (IC) chips.
| Element | Symbol | Atomic # | Atomic Mass | Classification | Electron Configuration |
|---|---|---|---|---|---|
| Carbon | C | 6 | 12.011 | Non-metal | [He]2s22p2 |
| Silicon | Si | 14 | 28.0855 | Metalloid | [Ne]3s23p2 |
| Germanium | Ge | 32 | 72.61 | Metalloid | [Ar]3d104s24p2 |
| Tin | Sn | 50 | 118.710 | Metal | [Kr]4d105s25p2 |
| Lead | Pb | 82 | 207.2 | Metal | [Xe]4f145d106s26p2 |
| Flerovium | Fl | 114 | 287 | Metal | [Rn]5f146d107s27p2 |
Carbon
Carbon is the fourth most abundant element on earth. It is of particular interest in organic chemistry, as it is the distinguishing feature of an organic compound. It is also considered the "backbone" of biology, as all life forms on earth are carbon-based. This is due to two important qualities of carbon: its small size and its unique electron configuration. Because carbon atoms are small, their p-orbital electrons overlap considerably and enable π bonds to form. Compare the molecular structures of CO2 and SiO2 below:
\(\ce{CO_2}\) has double bonds between carbon and oxygen atoms, whereas \(\ce{SiO2}\) has single bonds. The \(\ce{CO_2}\) molecule exists freely in the gas phase. The \(\ce{SiO_2}\) molecule, by contrast, always exists within a network of covalent bonds.
Carbon's electron configuration of allows it to form very stable bonds with oxygen and hydrogen. These bonds store an enormous amount of energy. The formation (fixation) and breakage (combustion) of these bonds in the carbon cycle facilitate earthly life:
- Carbon fixation: In photosynthesis, plants use energy from the sun and chlorophyll molecules to turn gaseous carbon dioxide from the atmosphere into simple carbohydrates like glucose:
\[\ce{6CO2 + 6H2O + energy → C6H12O6 + 6O2} \nonumber \]
- Carbon combustion: In aerobic respiration, plants and animals break carbohydrates down into carbon dioxide and water (as shown in the equation below) and use the energy released to fuel biological activities—growth, movement, etc. In addition, the combustion of carbohydrates found in fossil fuels provides energy needed for modern activities.
\[\ce{C6H12O6 + 6O2 → 6CO2 + 6H2O + energy} \nonumber \]
Next to sulfur, carbon is the element with the most allotropes. Carbon has three main solid state allotropes: graphite, diamond, and fullerenes (the most commonly known of which, buckminsterfullerene, is also known as a "buckyball"). These allotropes differ greatly in structure but are widely used in modern production.
Graphite and a diamond
Graphite has lubricating properties that make it extremely suitable for use in pencils. Because it is made up of planes of six-membered rings that can easily slide past one another, graphite glides easily and is hence used in combination with clay to form pencil "lead." Graphite is also used in a fibrous form for various plastics.
Carbon has very high melting and boiling points. Graphite is the most thermodynamically stable allotrope of carbon under ordinary conditions. In diamond, the more stable allotrope at extreme pressures (105 atm and up), each carbon atom is bonded to four others in a tetrahedral arrangement, resulting in the hardest naturally-occurring substance known. This hardness, combined with a good ability to dissipate heat, makes diamond and diamond film excellent materials in drill bits and other machine parts; however, the highest-quality natural diamonds are used mainly for jewelry, whereas lower-grade diamond or even synthetic diamond is used for industrial purposes.
Fullerenes (named after R. Buckminster Fuller) and nanotubes are a series of carbon allotropes in which carbon rings form more complex forms, including soccerball-like molecules (C60) and tubes resembling cylinders made of chicken wire. Graphene, a single carbon sheet with intriguing electronic properties, is the basis for these allotropes. Fullerenes occur when a certain percentage of hexagonal rings are assembled to form pentagonal rings, causing the sheet to contort into a roughly spherical "Buckyball." A carbon nanotube is simply graphene bent into a cylinder. Some of these allotropes are formed in the decomposition of graphite. Combustion can also yield alternate carbon forms. Heated coal without air forms coke. Similarly heated wood becomes charcoal as more volatile integrands are forced away.

There is a nearly innumerable amount of different carbon compounds, but several inorganic compounds are particularly important. Carbon monoxide (CO) is used for synthesizing other carbon compounds, reducing metal compounds to usable products, and in combination with other gases for fuel. Carbides, compounds of carbon and metals, are used in many industrial processes, often to stabilize other metal structures; calcium carbide is used to fabricate industrial chemical compounds, for example. Carbon disulfide and carbon tetrachloride are powerful solvents, (although since its classification as a carcinogen, CCl4 use has declined). Cyanide behaves similarly to halide ions, forming both a salt and an acid. Hydrocyanic acid (HCN) is a weak acid with an extremely low boiling point (room temperature in fact), and is used in plastic production. A cyanide dimer is called a cyanogen, and it is used in organic syntheses, fumigants, and rocket propellant.
Silicon
Although silicon plays a much smaller role in biology, it is the second most common element in the earth's crust (after oxygen) and is the backbone of the mineral world. It is classified as neither a metal or nonmetal, but a metalloid. Silicon is inert, primarily reacting with halogens. It may have functioned as a catalyst in the formation of the earliest organic molecules (Sadava 62). Plants depend on silicates (such as [SiO4]4-) to hold nutrients in the soil, where their roots can absorb them (Sadava 787). Silicon (primarily in the silica, SiO2, molecule) has been used for millennia in the creation of ceramics and glass. In more recent history, the name "Silicon Valley" attests to the element's importance in the computing industry— if carbon is the backbone of human intelligence, silicon is the backbone of artificial intelligence. Silicon is found in beach sand, and is a major component of concrete and brick.
![]()
Germanium
Germanium is a rare element used in the manufacture of semi-conductor devices. The physical and chemical properties of germanium are very similar to those of silicon. The semi-metal is found in coal, ore, and germanite. Germanium is gray-white in color and forms crystal structures.

Tin
Tin is a soft, malleable metal with a low melting point. It has two solid-state allotropes at regular temperatures and pressures, denoted α and β. At higher temperatures (above 13°C), tin exists as white tin, or β-tin, and is often used in alloys. At lower temperatures, tin can transform into gray tin, (α-tin); it loses its metallic properties and turns powdery. This causes the disintegration of items made from white tin alloys that have been exposed to the cold for long periods of time. The pipes in Europe's great pipe organs are a classic victim of this "tin pest." When a crystalline structure is broken, a "tin cry" is heard; this happens when a bar is bent. Gray tin is used to plate iron food cans to prevent them from rusting. Tin is malleable, ductile, and crystalline. It has 27 isotopes, 9 stable and 18 unstable. It is a superconductor at low temperatures. Tin reacts with bases, acid salts, and strong acids. Tin chlorides are good reducing agents and often used to reduce iron ores. Tin fluoride is often the anticavity "fluoride" additive in toothpastes.
Lead
Lead, (also known as plumbate), is similar to tin in that it is a soft, malleable metal with a low melting point. It was formerly widely used in water and sewage pipes, lending its Latin name (plumbum ) to the terms "plumber" and "plumbing." Lead is toxic to humans, especially children. Even low levels of exposure can cause nervous system damage and can prevent proper production of hemoglobin (the molecule in red blood cells responsible for carrying oxygen through the body). Because of this, there has been a concerted effort to reduce public exposure to lead, including an emphasis on using unleaded gasoline and unleaded paint. Lead is typically stable in an oxidation state of +2 or +4. Its oxides have many industrial uses as oxidizing agents, such as cathodes in lead-acid storage cells.

Flerovium
Flerovium (Fl) is also known as Element 114. It was discovered in 1998 by scientists in Dubna. It is radioactive and very short-lived.
References
- Petrucci et al. General Chemistry: Principles & Modern Applications, 9th Edition. New Jersey: Pearson Education, Inc., 2007.
- Sadava et al. Life: The Science of Biology, 8th Edition. Sunderland, MA: Sinauer Associates, Inc., 2008.
Problems
Here are some questions to test your understanding of this material.
- Recall the metallic properties. What makes tin and lead "poor" metals?
- What makes graphite such a good material for pencil lead?
- What makes diamonds so hard?
- Why is tin used to plate iron cans?
- Why are +2 and +4 the most common oxidation states of metals in this group?
Solution
- They do not conduct heat or electricity very well.
- It is composed of flat sheets, which are weakly bonded to one another, so they easily slide past each other and rub off on paper.
- Each carbon atom forms bonds with four other carbon atoms in a tetrahedral crystal. This arrangement is extremely strong.
- The tin plating prevents the iron can from oxidizing (rusting).
- Because the valence electron configuration is \(ns^2np^2\), the atoms tend to lose either all four outer shell electrons (resulting in a charge of +4) or, because of the inert pair effect, they may lose only the s electrons (resulting in a charge of +2).
Contributors and Attributions
- Elizabeth Sproat, Jessica Lin, Vancy Wong

