24.16.1: Organolead Chemistry
- Page ID
- 85475
Tetraethyllead
Like mercury, lead is primarily obtained from its sulfide ore, in this case Galena, PbS, yet once again there are quite a number of other minerals containing lead. In terms of natural abundance it exists at about 14 ppm in the Earth's crust (37th compared to O), however it has become well known due to its ease of extraction and the number of uses with technical importance.
galena, PbS
Lead was probably discovered around 6500 BC in Turkey and by 300 BC the Romans had lead smelters in operation. The toxicity of lead was recorded by the Greeks as early as 100BC. A report from 2BC noted that:
"the drinking of lead causes oppression to the stomach, belly and intestines with wringing pains; it suppresses the urine, while the body swells and acquires and unsightly leaden hue".
The possible hazards associated with the use of lead piping in water systems was recognized as long ago as the first century BC and it has even been suggested that the "decline of the Roman Empire" might have been ascribed to the use of lead acetate as an additive to sweeten wine. It is somewhat surprising therefore that the first legislation controlling the industrial hazards of lead industries was not introduced until 1864.
Note that Dr. Wilton Turner (born in Clarendon, Jamaica in the early 1800's) wrote on the inappropriate use of lead in sugar and rum production while running a rum distillery in Guyana. One advocate said he had fed lead to dogs and guinea pigs for several weeks and seen no adverse affects in fact the guinea pigs were stolen which he thought was because they looked so fat and healthy!
An examination (in the early 1970's) of the annual snow strata in Northern Greenland and Poland revealed most elegantly that levels in air-borne lead had increased significantly since the Industrial Revolution and very sharply since 1940. Considering that 40-50% can be absorbed by inhalation compared to only 5-10% through ingestion this was cause for concern.
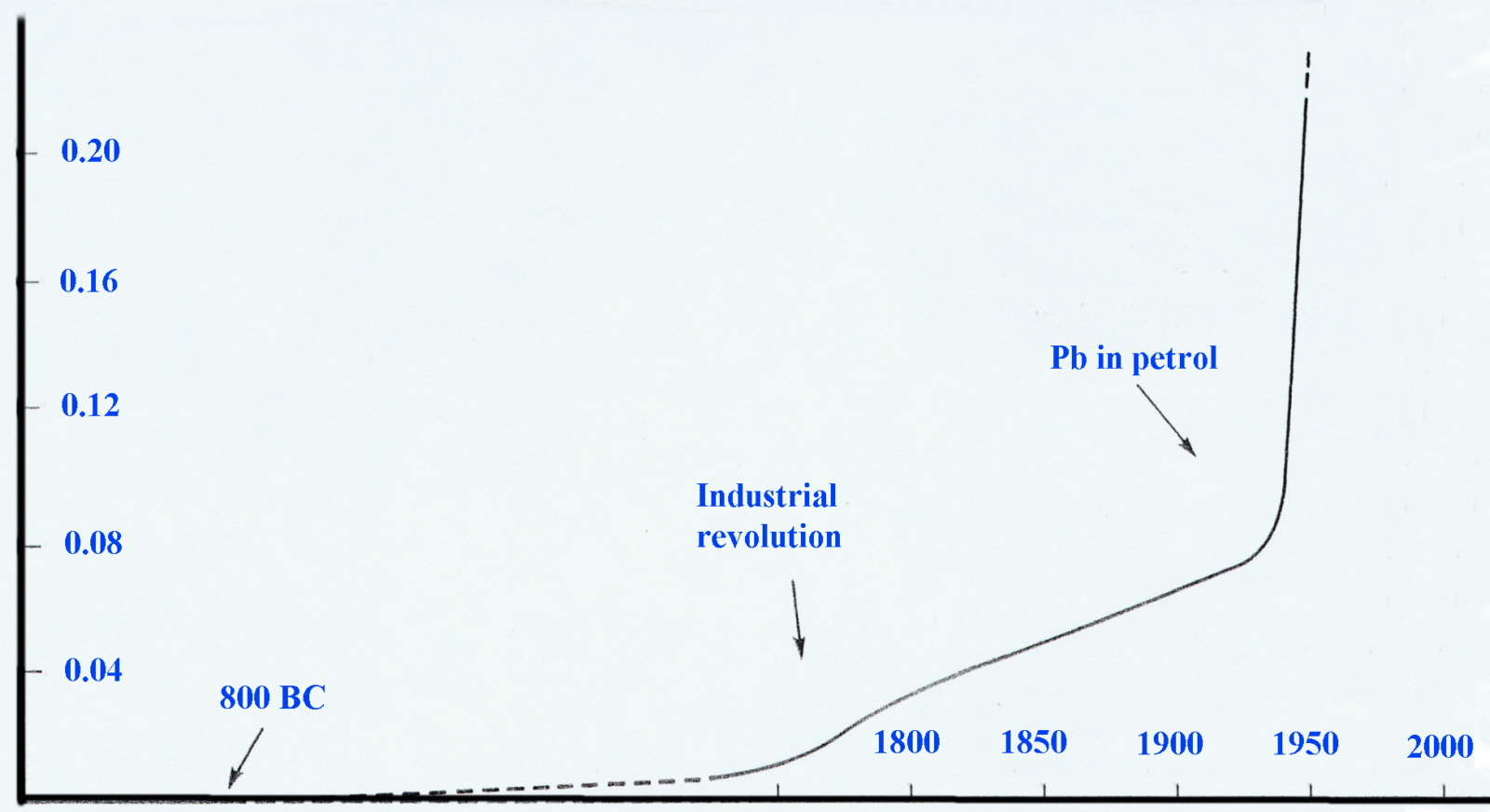
Lead content in North Greenland snow layers (µg per kg)
Leaded gasoline was an economic success from 1926 until 1976, and in fact, its discovery by Thomas Midgley at Charles Kettering's General Motors laboratory was among the most celebrated achievements of automotive engineering. It was often portrayed as the result of genius, luck and a great deal of hard work. It is now considered to be a catastrophic failure and is banned for environmental and public health reasons. {There are still a few countries selling petrol with lead additives.} Even more surprising is that the use of ethanol in fuel was already well established by the time tetraethyllead was introduced as an additive.
Tetraethyllead was supplied for mixing with raw gasoline in the form of "ethyl fluid", which was Et4Pb blended together with the lead scavengers 1,2-dibromoethane and 1,2-dichloroethane. "Ethyl fluid" also contained a reddish dye to distinguish treated from untreated gasoline and discourage the use of leaded gasoline for other purposes such as cleaning.
Ethyl fluid was added to gasoline in the ratio of 1:1260, usually at the refinery. The purpose was to increase the fuel's octane rating. A high enough octane rating is required to prevent premature detonations known as engine knocking ("knock" or "ping"). Antiknock agents allow the use of higher compression ratios for greater efficiency and peak power. The formulation of "ethyl fluid" was:
- Tetraethyllead 61.45%
- 1,2-Dibromoethane 17.85%
- 1,2-Dichloroethane 18.80%
- Inert materials and dye 1.90%
Effect on Health
Humans have been mining and using this heavy metal for thousands of years, poisoning themselves in the process. Although lead poisoning is one of the oldest known work and environmental hazards, the modern understanding of the small amount of lead necessary to cause harm did not come about until the latter half of the 20th century. No safe threshold for lead exposure has been discovered, that is, there is no known amount of lead that is too small to cause the body harm.
Lead pollution from engine exhaust is dispersed into the air and into the vicinity of roads and easily inhaled. Lead is a toxic metal that accumulates and has subtle and insidious neurotoxic effects especially at low exposure levels, such as low IQ and antisocial behavior. It has particularly harmful effects on children. These concerns eventually led to the ban on Et4PB in automobile gasoline in many countries. For the entire U.S. population, during and after the Et4PB phaseout, the mean blood lead level dropped from 13 µg/dL in 1976 to only 3 µg/dL in 1991. The U.S. Centers for Disease Control considered blood lead levels "elevated" when they were above 10 µg/dL. Lead exposure affects the intelligence quotient (IQ) such that a blood lead level of 30 µg/dL is associated with a 6.9-point reduction of IQ, with most reduction (3.9 points) occurring below 10 µg/dL.
Also in the U.S., a statistically significant correlation has been found between the use of Et4PB and violent crime: taking into account a 22-year time lag, the violent crime curve virtually tracks the lead exposure curve. After the ban on Et4PB, blood lead levels in U.S. children dramatically decreased.
Even though leaded gasoline is largely gone in North America, it has left high concentrations of lead in the soil adjacent to all roads that were constructed prior to its phaseout. Children are particularly at risk if they consume this, as in cases of pica.
Note as well the work done in 1995 by ICENS on the problem of the old disused lead mine and tailings that affected school children in Kintyre. Over 40 cases were detected with unacceptable levels. ICENS at that time cleaned the community and sought to educate residents about the dangers. A continuation of the research done in Kintyre was to test 628 children at 17 basic schools across the island. Children at a number of basic schools in Kingston and St Catherine were discovered with blood lead levels as low as 45 µg/dL and as high as 60. In two of the cases, children had lead levels of 130 and 202. At this level, they would likely die from the poisoning if untreated.
| Molecular formula | C8H20Pb |
|---|---|
| Molar mass | 323.44 g mol-1 |
| Appearance | Colourless, viscous liquid |
| Density | 1.653 g/mL (20 °C) |
| Melting point | -136 °C |
| Boiling point | 84-85 °C/15 mm Hg |
| Solubility in water | Insoluble |
Laboratory Preparation:
The industrial preparation of tetraethyllead was from the reaction below:
~373K in an autoclave
\[\ce{4 NaPb + 4 EtCl → Et4Pb + 3 Pb + 4 NaCl}\]
alloy or by electrolysis of NaAlEt4 or EtMgCl using a Pb anode. Laboratory syntheses of R4Pb compounds in general include the use of Grignard reagents or organolithium compounds.
\[\ce{2 PbCl2 + 4 RLi → R4Pb + 4LiCl + Pb}\]
in ether
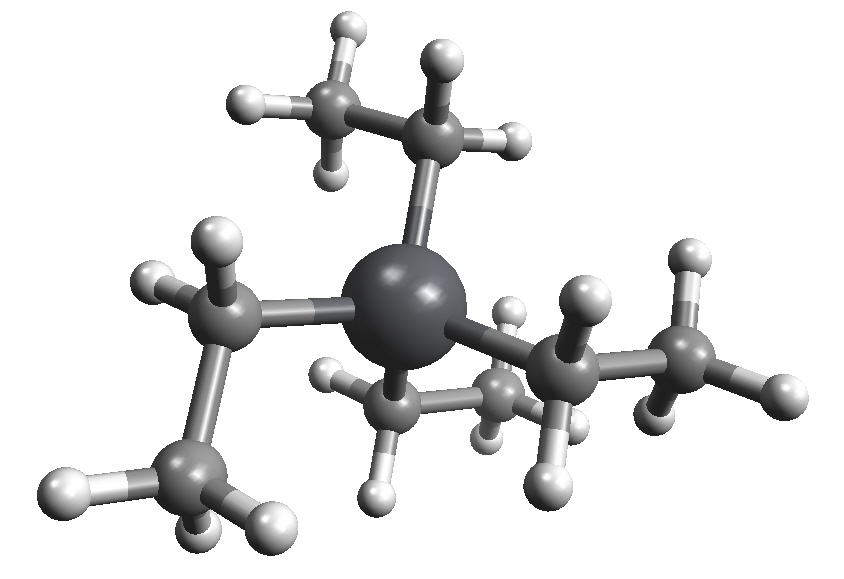
tetraethyllead
lead timeline
Tetraethyllead
"ethyl" articles

