24.12: Carbene and Carbyne Complexes
- Page ID
- 34576
http://nptel.ac.in/courses/104101006...mod13/lec2.pdf
http://nptel.ac.in/courses/104101006/
http://mcindoe.pbworks.com/w/page/20...67/Alkylidynes
http://mcindoe.pbworks.com/w/page/20651670/FrontPage
http://www.massey.ac.nz/~gjrowlan/adv/lct6.pdf
http://www.f.u-tokyo.ac.jp/~kanai/se.../Hartwig13.pdf
In this lecture you will learn the following
- The metal−ligand multiple bonding and their relevance in.
- The Fischer type carbene complexes.
- The Schrock type carbene complexes.
The organometallic compounds containing metal−ligand multiple bonds of the types, M=X and M≡X (X = C, N, O) are of current interest as they are valuable intermediates in many important catalytic cycles. In this regard, considerable attention has been paid towards developing an understanding of the metal−ligand multiply bonded systems like that of the metal carbene LnM=CR2 type complexes and of the metal carbyne LnM≡CR type complexes. A detailed account of the metal−carbene complexes is presented in this chapter.
Metal-carbene Complexes
Carbenes are highly reactive hexavalent species that exist in two spin states, i.e. (i) in a singlet form (), in which two electrons are paired up and (ii) in a triplet form (), in which the two electrons remain unpaired. Of the two, the singlet form is the more reactive one. The instability of carbene accounts for its unique reactivity like that of the insertion reaction, which has aroused significant interest in recent years. The singlet carbene and the triplet carbene bind differently to metals, with the singlet one yielding Fischer type carbene complexes while the triplet one yielding Schrock type carbene complexes (Figure 1).
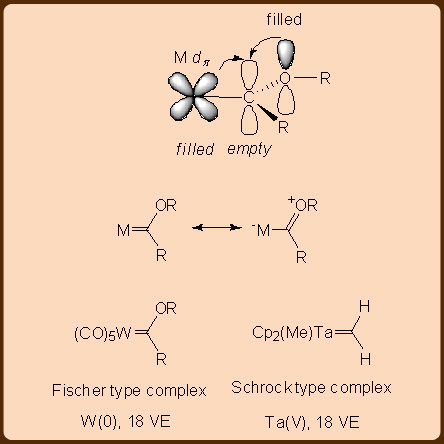
The LnM=CR2 type Fischer carbene complexes comprise of two dative covalent interactions that include (i) a LnM←CR2 type ligand to metal σ−donation and (ii) a LnM→CR2 type metal to ligand π−back donation. The Fischer type carbene complexes are usually formed with metal centers at a low oxidation state. These are also commonly observed for the more electron rich late−transition metals that participate in the LnM→CR2 type metal to ligand π−back donation. Another characteristic of the Fischer type carbene complex is the presence of the heteroatom substituents like R = OMe or NMe2 on the carbene CR2 moiety which makes the carbene carbon significantly cationic (δ+) to facilitate the LnM→CR2 type metal to ligand π−back donation.

Similarly, the LnM=CR2 type Schrock carbene complexes comprise of two covalent interactions that involve one electron donation towards the σ−bond from each of the metal LnM and the carbene CR2 fragments. Schrock carbene complexes are thus formed with the metal centers having high oxidation state and are usually observed for electron deficient early−transition metals (Figure 2).
Carbene complexes can be prepared by the following methods.
- by the reaction with electrophiles

- by H−/H+ abstraction reactions as shown below

- from low−valent metal complexes

Because of the electronically different metal−ligand interaction that exist between the LnM and the carbene CR2 moiety, the reactivity of Fischer and Schrock carbene complexes are completely different. For example, the Fischer type carbene complexes undergo attack by nucleophiles at its carbene−C center.

The Schrock type carbene complexes on the other hand undergo attack by electrophiles at its carbene−C center.
Summary
The metal−ligand multiple bonding is of significant interest as many of the compounds containing such bonds are important intermediates in various catalytic cycles. The metal−ligand doubly bonded carbene systems can exist in two varieties like the Fischer type and the Schrock type carbene complexes. Due to their different electronic structures, the reactivities of these Fischer type and the Schrock type carbene complexes differ significantly, with the former undergoing nucleophilic attack while the later undergo electrophilic attack at their respective carbene−C centers.
Metal-carbyne complexes
The metal−ligand multiply bonded systems even extended beyond the doubly bonded Fischer and the Schrock carbenes to the triply bonded LnM≡CR type Fischer carbyne and the Schrock carbyne complexes. Similar to carbene that exists in a singlet and a triplet spin state, the carbyne also exists in two other spin states i.e in a doublet and a quartet form.
Upon binding to the metal in its doublet spin state as in the Fischer carbene system, the carbyne moiety donates two electrons via its sp hybridized lone pair containing orbital to an empty metal d orbital to yield a LnM←CR type ligand to metal dative bond. It also makes a covalent π−bond through one of its singly occupied pz orbital with one of the metal d orbitals. The carbyne−metal interaction consist of two ligand to metal interactions namely a dative one and a covalent one that together makes the carbyne moiety a LX type of a ligand. In addition to these two types of ligand to metal bonding interactions, there remains an empty py orbital on the carbyne−C atom that can accommodate electron donation from a filled metal d orbital to give a metal to ligand π−back bonding interaction (Figure 1).

Analogously, in the quartet carbyne spin state in the Schrock carbyne systems three covalent bonds occur between the singly occupied sp, pyand pz orbitals of carbyne−C moiety with the respective singly occupied metal d orbitals (Figure 1).
Similar to what has been observed earlier in the case of the Fischer carbenes and Schrock carbenes, the Fischer carbyne complexes are formed with metal centers in lower oxidation states for e.g. as in Br(CO)4W≡CMe, while the Schrock carbyne complexes are formed with metals in higher oxidation state, e.g. as in (t−BuO)3W≡Ct−Bu.
Carbyne complexes can be prepared by the following methods.
- The Fischer carbyne complexes can be prepared by the electrophilic abstraction of a methoxy group from a methoxy methyl substituted Fischer carbene complex.

- Schrock carbynes can be prepared by the deprotonation of a α−CH bond of a metal−carbene complex.

- by an α−elimination reaction on a metal−carbene complex

- by metathesis reaction

The reactivities of Fischer and the Schrock carbynes mirror that of the Fischer and Schrock carbenes. For example, the Fischer carbyne undergo nucleophilic attack at the carbyne−C atom while the Schrock carbyne undergo electrophilic attack at the carbyne−C atom.
Summary
The theme of metal−ligand multiple bonding extends beyond the doubly bonded Fischer and the Schrock carbene systems to even triply bonded Fischer and the Schrock carbyne systems. The carbyne moieties in these Fischer and the Schrock carbyne systems respectively exist in a doublet and a quartet spin state. The carbyne complexes are generally prepared from the respective carbene analogues by the abstraction of alkoxy (OR), proton (H+), hydride (H−) moieties, the α−elimination reactions and the metathesis reactions. The reactivity of the Fischer and the Schrock carbyne complexes parallel the corresponding Fischer and the Schrock carbene counterparts with regard to their reactivities toward electrophiles and nucleophiles.

