21.6A: The Metal
- Page ID
- 34435
The discovery of vanadium is attributed to Andres Manuel del Rio (a Spanish mineralogist working in Mexico City) who prepared a number of salts from a material contained in "brown lead" around 1801. Unfortunately, the French chemist Collett-Desotils incorrectly declared that del Rio's new element was only impure Chromium. Del Rio thought himself to be mistaken and withdrew his claim. The element was rediscovered in 1830 by the Swedish chemist Nils Gabriel Sefström who named it after the Norse goddess Vanadis, the goddess of beauty and fertility.
Metallic vanadium was not isolated until 1867 when Sir Henry Enfield Roscoe (1833-1915), Professor of Chemistry at Owens College (later the University of Manchester) from 1857 to 1885, reduced vanadium chloride (VCl5) with gaseous hydrogen to give vanadium metal and HCl.
Properties of vanadium
An excellent site for finding the properties of the elements, including vanadium is at ![]()
Introduction
Vanadium has been found to play a number of roles in biological systems. It is present in certain vanadium dependent haloperoxidase and nitrogenase enzymes.

A tunicate (Clavelina Puertosecensis) discovered near Discovery Bay, Jamaica
Many sea squirts, such as Ciona Intestinalis accumulate vanadium in very high concentration, although the reason is not known.

The mushroom Amanita muscaria accumulate vanadium in the form of a coordination complex called amavadin, whose function is still unknown.
A number of vanadium complexes have been shown to alleviate many of the symptoms of diabetes in both in vitro and in vivo (in rats and mice) studies. These complexes are being studied as potential alternatives to insulin therapy.
Vanadium Halides
| Formula | Colour | MP | BP | m (BM) | Structure |
|---|---|---|---|---|---|
| VF5 | white | 19.5 | 48.3 | 0 | trigonal bipyramid in gas phase |
Preparations:
Prepared by reaction of V with F2 in N2 or with BrF3 at 300C.
In the solid state it is an infinite chain polymer with cis-fluoride bridging.
| Formula | Colour | MP | BP | m (BM) | Structure |
|---|---|---|---|---|---|
| VF4 | lime-green | 100 (a) | - | 1.68 | - |
| VCl4 | red-brown | -25.7 | 148 | 1.61 | tetrahedral (monomeric) |
| VBr4 | purple | -23d | - | - | - |
(a) sublimes with decomposition at 100 C.
Preparations:
VCl4 is prepared by reaction of V with chlorinating agents such as Cl2, SOCl2, COCl2 etc.
Reaction of VCl4 with HF in CCl3F at -78C gives VF4.
Vanadium Oxides and Aqueous Chemistry
| Formula | Colour | Common name | Oxidation State | MP | V-O distance (pm) |
|---|---|---|---|---|---|
| V2O5 | brick-red | pentoxide | V5+ | 658 | 158.5-202 |
| V2O4 | blue | dioxide | V4+ | 1637 | 176-205 |
| V2O3 | grey-black | sesquioxide | V3+ | 1967 | 196-206 |
Preparations:
V2O5 is the final product of the oxidation of V metal, lower oxides etc.
Aqueous Chemistry very complex:
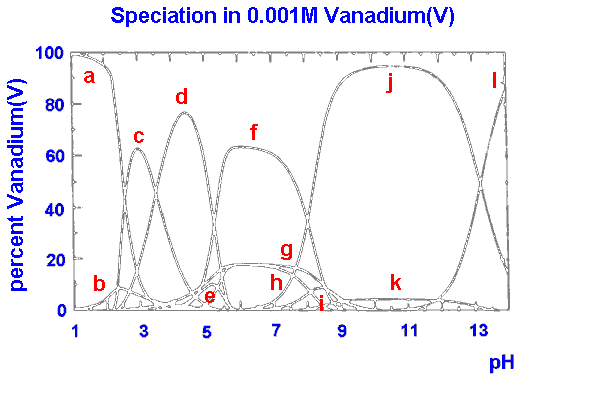 |
a VO2+ b VO(OH)3 c V10O26(OH)24- d V10O27(OH)5- e V10O286- f V3O93- g VO2(OH)2- h V4O124- i V2O6(OH)3- j VO3(OH)2- k V2O74- l VO43- |
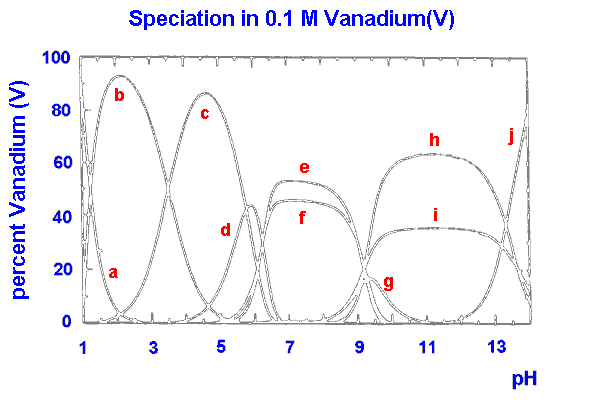 |
a VO2+ b V10O26(OH)24- c V10O27(OH)5- d V10O286- e V4O124- f V3O93- g V2O6(OH)3- h V2O74- i VO3(OH)2- j VO43- |
In alkaline solution,
VO43- + H+ → HVO42-
2HVO42- → V2O74- + H2O
HVO42- + H+ → H2VO4-
3H2VO4- → V3O93- + 3H2O
4H2VO4- → V3O124- + 4H2O
In acidic solution,
10V3O93- + 15H+ → 3HV10O285- + 6H2O
H2VO4- + H+ → H2VO4
HV10O285- + H+ → H2V10O284-
H3VO4 + H+ → VO2+ + 2H2O
H2V10O284- + 14H+ → 10VO2+ + 8H2O
VO(H2O)4SO4
The crystal structure of this salt was first determined in 1965. The V=O bond length was 159.4 pm, the aquo group trans to this had the longest V-O bond length (228.4pm) and the equatorial bond lengths were in the range 200.5-205.6 pm. Note that SO42- was coordinated in an equatorial position.
The IR stretching frequency for the V=O in vanadyl complexes generally occurs at 985 +/- 50 cm-1.
Redox properties of oxovanadium ions:
VO2+ + 2H+ + e- → VO2+ + H2O E=1.0 V
VO2+ + 2H+ + e- → V3+ + H2O E=0.34 V
References
- "Inorganic Chemistry", 3rd Edition, Catherine Housecroft, Alan G. Sharpe, Publisher: Prentice Hall
- "Complexes and First-Row Transition Elements", D. Nicholls
- "Basic Inorganic Chemistry", F.A. Cotton, G. Wilkinson and P.L. Gaus
- "Advanced Inorganic Chemistry", F.A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann
- "Chemistry of the Elements", Greenwood and Earnshaw
- "Hydrolysis of Cations", Baes and Messmer
Contributors and Attributions
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)

