19.8B: Structural Isomerism - Hydration Isomers
- Page ID
- 34365
A very similar type of isomerism results from replacement of a coordinated group by a solvent molecule (Solvate Isomerism), which in the case of water is called Hydrate Isomerism. The best known example of this occurs for chromium chloride (\(CrCl_3 \cdot 6H_2O\)) which may contain 4, 5, or 6 coordinated water molecules (assuming a coordination number of 6). The dot here is used essentially as an expression of ignorance to indicate that, though the parts of the molecule separated by the dot are bonded to one another in some fashion, the exact structural details of that interaction are not fully expressed in the resulting formula. Using Alfred Werner’s coordination theory that indicates that several of the water molecules are actually bonded directly (via coordinate covalent bonds) to the central chromium ion. In fact, there are several possible compounds that use the brackets to signify bonding in the complex and the the dots to signify "water molecules that are not bound to the central metal, but are part of the lattice:
- \([CrCl_2(H_2O)_4]Cl \cdot 2H_2O\): bright-green colored
- \([CrCl(H_2O)_5]Cl_2 \cdot H_2O\): grey-green colored
- \([Cr(H_2O)_6]Cl_3\): violet colored
These isomers have very different chemical properties and on reaction with \(AgNO_3\) to test for \(Cl^-\) ions, would find 1, 2, and 3 \(Cl^-\) ions in solution, respectively.
Upon crystallization from water, many compounds incorporate water molecules in their crystalline frameworks. These "waters of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation. In the first two hydrate isomers, there are water molecules that are artifacts of the crystallization and occur inside crystals. These water of crystallization is the total weight of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio.
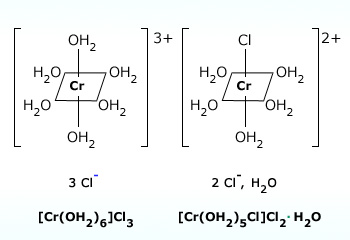
The \([Cr(H_2O)_6]Cl_3\) hydrate isomer (left) is violet colored and the \([CrCl(H_2O)_5]Cl_2 \cdot H_2O\) hydrate isomer is green-grey colored.
What are "Waters of Crystallization"?
A compound with associated water of crystallization is known as a hydrate. The structure of hydrates can be quite elaborate, because of the existence of hydrogen bonds that define polymeric structures. For example, consider the aquo complex \(NiCl_2 \cdot 6H_2O\) that consists of separated trans-[NiCl2(H2O)4] molecules linked more weakly to adjacent water molecules. Only four of the six water molecules in the formula are bound to the nickel (II) cation, and the remaining two are waters of crystallization as the crystal structure resolves.
6forFeCoNi.png?revision=1&size=bestfit&width=388&height=314)
Structure of \(NiCl_2 \cdot 6H_2O\) salt with chlorine atoms (green), water molecules (red), and Ni metals (blue) indicated. (CC BY-SA 4.0; Smokefoot).
Water is particularly common solvent to be found in crystals because it is small and polar. But all solvents can be found in some host crystals. Water is noteworthy because it is reactive, whereas other solvents such as benzene are considered to be chemically innocuous.

