10.4D: Reactivity
- Page ID
- 34059
The position of H in the Periodic Table
In some respects, H does not seem to have a perfect position in the Periodic Table and so many designers have it in more than one position, e.g. in Group 1 or Group 17 and even in Group 14.
Ionization energy of hydrogen
Hydrogen has a single outer electron, like the alkali metals, but they all form positive ions quite readily whereas hydrogen has little tendency to do so. Hydrogen often tends to share its electron with nonmetals rather than losing it to them.
The first ionization energies for H, Li, Na and K are 1312, 520.2, 495.8 and 418.8 kJmol-1. The high IE for H (even bigger than for Xe) can be attributed to the very small size of the atom and the strong attractive force between the proton and electron.
Xe(g) → Xe+(g) + e ΔH⦵ = 1170 kJmol-1
The free proton can only be obtained under extreme conditions such as by an electric arc or in a discharge tube and even then only exists for about half a second. H+ can be found in solvated form where the solvation energy provides the energy needed to overcome the very high ionization energy. Examples are in ammonia, alcohol or water with species like NH4+, ROH2+ and H3O4+ being formed.
The hydrated proton (H3O4+) will be covered in Lecture 6 on acid-base chemistry.
Electron affinity of hydrogen
Hydrogen, like the halogens, exists as diatomic molecules and H atoms have electron configurations with one electron short of a filled outer shell hence the idea of placing H in Group 17. However unlike the halogens with large EA values, the EA for hydrogen is quite small. The formation of H- is much less favourable than the formation of a chloride ion, as seen from the thermodynamic profiles below and it is rare whereas halide ions are common and stable. In addition H has a lower electronegativity value than any of the halogens.
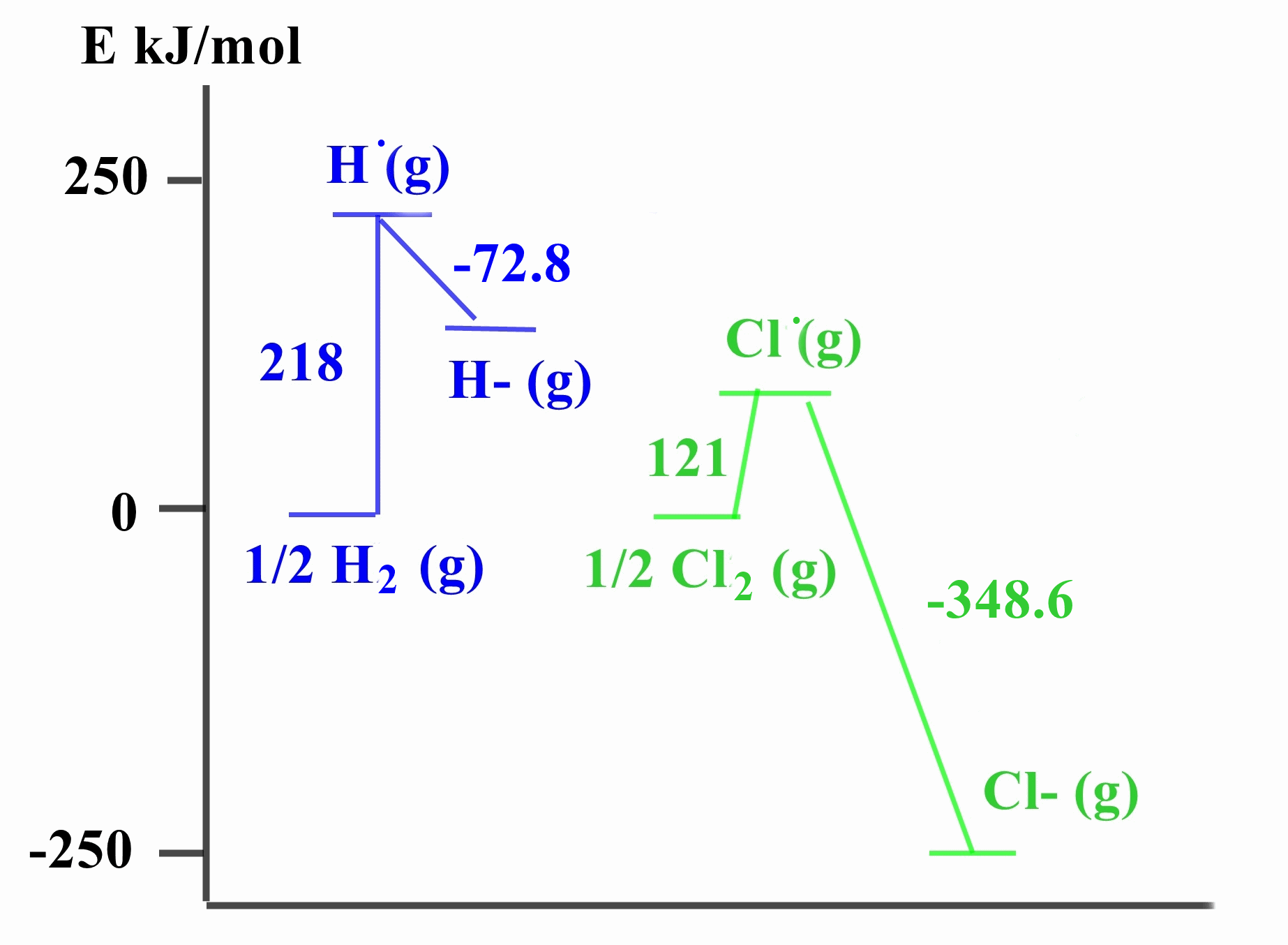 Much more energy is required as well to break the H-H bond compared to the Cl-Cl bond where the steps for comparison are:
½H2 (g) → H. (g) ΔH = 218 kJmol-1
Much more energy is required as well to break the H-H bond compared to the Cl-Cl bond where the steps for comparison are:
½H2 (g) → H. (g) ΔH = 218 kJmol-1H. (g) + e → H- (g) ΔH = -72.8 kJmol-1
so overall for hydrogen
½H2 (g) + e → H- (g) ΔH = +145.2 kJmol-1
and
½Cl2 (g) → Cl. (g) ΔH = 121 kJmol-1
Cl. (g) + e → Cl- (g) ΔH = -348.6 kJmol-1
overall for chlorine
½Cl2 (g) + e → Cl- (g) ΔH = -227.6 kJmol-1 As a result, only the most active elements, whose Ionization Energies are low, can form ionic hydrides, e.g. NaH.
The covalent radius for H is 37 pm and the estimated radius for H- is ~140 pm indicating a substantial increase. This comes about as a result of the interelectronic repulsion when a second electron is added to the 1s atomic orbital. All the alkali metal hydrides crystallize with the NaCl-type structure and are all considered ionic. They are sometimes called "saline" hydrides.

