10.4C: Synthesis and Uses
- Page ID
- 34058
In the laboratory, H2 can be prepared by the action of a dilute non-oxidizing acid on a reactive metal such as zinc, with a Kipp's apparatus.
\[\ce{Zn + 2H (aq)^{+} \rightleftharpoons Zn (aq) ^{2+} + H2}\nonumber \]
Aluminium can produce H2 upon treatment with bases:
\[\ce{2Al + 6 H2O + 2 OH^{-} \rightleftharpoons 2 Al(OH)4^{-} + 3 H2}\nonumber \]
The electrolysis of water is another simple method of producing hydrogen. A low voltage current is passed through the water, and gaseous dioxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or other inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used versus energetic value of hydrogen produced) is in the range 80-94%.
\[\ce{2 H2O(l) \rightleftharpoons 2 H2(g) + O2(g)}\nonumber \]
In 2007, it was discovered that an alloy of aluminum and gallium in pellet form added to water could be used to generate hydrogen. The process creates alumina, but the expensive gallium, which prevents the formation of an oxide skin on the pellets, can be re-used. This has important potential implications for a hydrogen economy, as hydrogen could be produced on-site without the need of being transported.
Industrial preparation of hydrogen
Steam reforming is a method for producing hydrogen, carbon monoxide or other useful products from hydrocarbon fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature with the fossil fuel.
At high temperatures (700 - 1100 °C) and in the presence of a metal-based catalyst (nickel), steam reacts with methane to yield carbon monoxide and hydrogen.
\[\ce{CH4 + H2O → CO + 3 H2}\nonumber \]
In order to produce more hydrogen from this mixture, more steam is added and the water gas shift reaction is carried out:
\[\ce{CO + H2O → CO2 + H2}\nonumber \]
The mixture of CO and H2 is called "synthesis gas or syngas". Syngas is used as an intermediate in producing synthetic petroleum for use as a fuel or lubricant via the Fischer-Tropsch process and previously the Mobil methanol to gasoline process.
Enzymatic route from xylose
In 2013 a low-temperature, 50 °C, atmospheric-pressure, enzyme-driven process to convert xylose into hydrogen with nearly 100% of the theoretical yield was announced. The process employed 13 enzymes, including a novel polyphosphate xylulokinase (XK).
It was noted that: "Approximately 50 million metric tons of dihydrogen are produced annually from nonrenewable natural gas, petroleum, and coal. H2 production from water remains costly. Technologies for generating H2 from less costly biomass, such as microbial fermentation, enzymatic decomposition, gasification, steam reforming, and aqueous phase reforming, all suffer from low product yields."
Applications of Hydrogen
Large quantities of H2 are used by the petroleum and chemical industries. The largest application of H2 is for the processing ("upgrading") of fossil fuels, and in the production of ammonia. The key consumers of H2 in the petrochemical plant include hydrodealkylation, hydrodesulfurization, and hydrocracking. H2 has several other important uses. H2 is used as a hydrogenating agent, particularly in increasing the level of saturation of unsaturated fats and oils (found in items such as margarine), and in the production of methanol. It is similarly the source of hydrogen in the manufacture of hydrochloric acid. H2 is used as a reducing agent of metallic ores.
Nitrogen is a strong limiting nutrient in plant growth. Carbon and oxygen are also critical, but are more easily obtained by plants from soil and air. Even though air is 78% nitrogen, atmospheric nitrogen is nutritionally unavailable because nitrogen molecules are held together by strong triple bonds. Nitrogen must be 'fixed', i.e. converted into some bioavailable form, through natural or man-made processes. It was not until the early 20th century that Fritz Haber developed the first practical process to convert atmospheric nitrogen to ammonia, which is nutritionally available.
Fertilizer generated from ammonia produced by the Haber process is estimated to be responsible for sustaining one-third of the Earth's population. It is estimated that half of the protein within human beings is made of nitrogen that was originally fixed by this process; the remainder was produced by nitrogen fixing bacteria and archaea.
Dozens of chemical plants worldwide produce ammonia, consuming more than 1% of all man-made power. Ammonia production is thus a significant component of the world energy budget. Modern ammonia-producing plants depend on industrial hydrogen production to react with atmospheric nitrogen using a magnetite catalyst or over a promoted Fe catalyst under high pressure (100 standard atmospheres (10,000 kPa)) and temperature (450 °C) to form anhydrous liquid ammonia. This step is known as the ammonia synthesis loop (also referred to as the Haber-Bosch process):
\[\ce{3 H2 + N2 \rightleftharpoons 2 NH3} \,\, ΔH = -92.4\, kJ\,mol^{-1}\nonumber \]
Nitrogen (N2) is very unreactive because the molecules are held together by strong triple bonds. The Haber process relies on catalysts that accelerate the cleavage of this triple bond.
At room temperature, the equilibrium is strongly in favor of ammonia, but the reaction doesn't proceed at a detectable rate. Thus two opposing considerations are relevant to this synthesis. One possible solution is to raise the temperature, but because the reaction is exothermic, the equilibrium quickly becomes quite unfavourable at atmospheric pressure. Low temperatures are not an option since the catalyst requires a temperature of at least 400 °C to be efficient. By increasing the pressure to around 200 atm the equilibrium concentrations are altered to give a profitable yield.
The reaction scheme, involving the heterogeneous catalyst, is believed to involve the following steps:
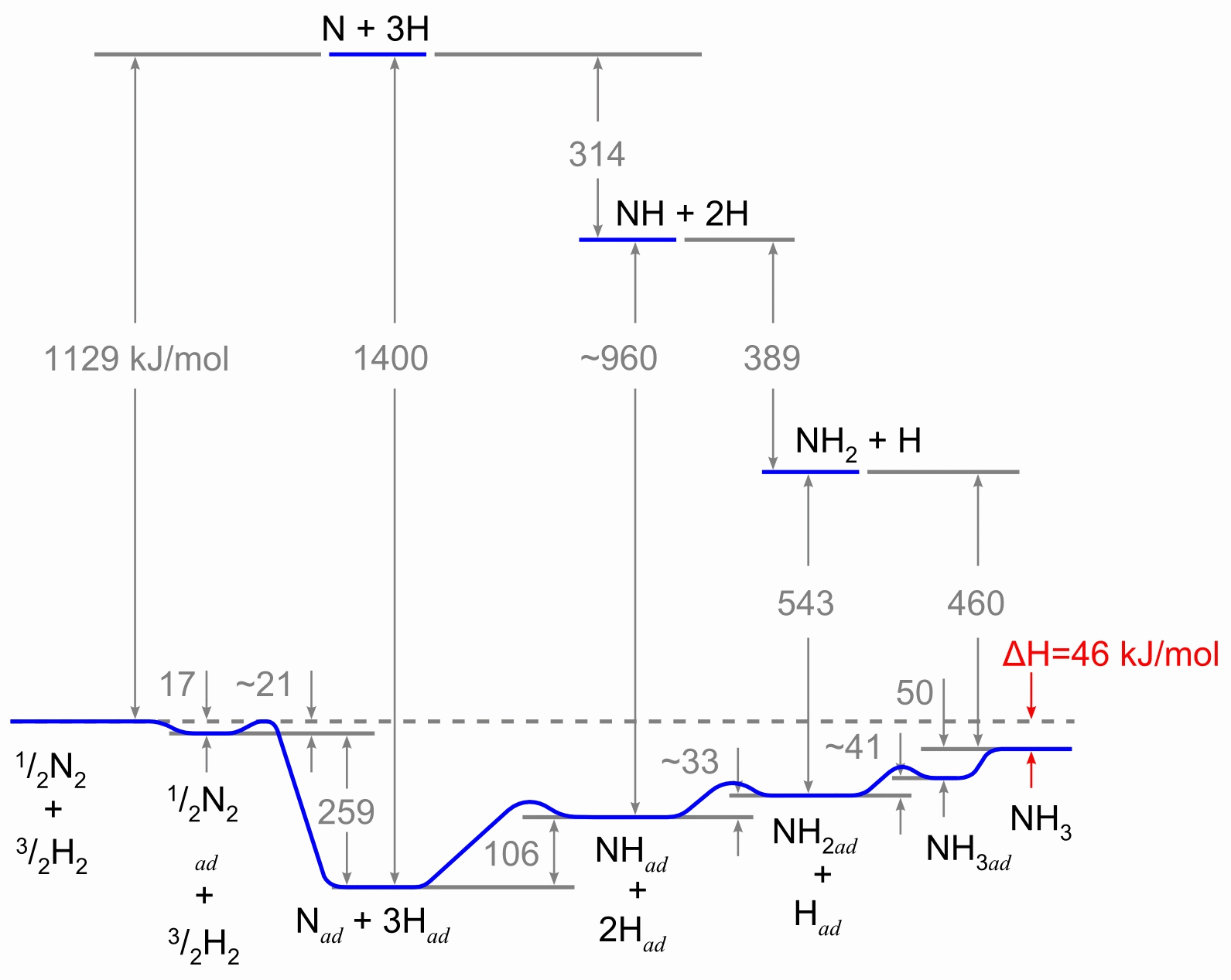
- N2 (g) → N2 (adsorbed)
- N2 (adsorbed) → 2 N (adsorbed)
- H2(g) → H2 (adsorbed)
- H2 (adsorbed) → 2 H (adsorbed)
- N (adsorbed) + 3 H (adsorbed) → NH3 (adsorbed)
- NH3 (adsorbed) → NH3 (g)
Reaction 5 actually consists of three steps, forming NH, NH2, and then NH3. Experimental evidence suggests that reaction 2 is the slow, rate-determining step. This is not unexpected given that the bond broken, the nitrogen triple bond, is the strongest of the bonds that must be broken.


