9.9: Superacids
- Page ID
- 33825
The term superacid was originally coined by James Bryant Conant in 1927 to describe acids that were stronger than conventional mineral acids.[1] George A. Olah prepared the so-called magic acid, so-named for its ability to attack hydrocarbons, by mixing antimony pentafluoride (SbF5) and fluorosulfonic acid (FSO3H). The name was coined after a candle was placed in a sample of magic acid. The candle dissolved, showing the ability of the acid to protonate hydrocarbons, which under aqueous acidic conditions cannot be protonated.
At 140 °C , FSO3H–SbF5 converts methane into the tertiary-butyl carbocation, a reaction that begins with the protonation of methane:[2]
- \(CH_4 + H^+ \rightarrow CH^+_5\)
- \(CH^+_5 \rightarrow CH^+_3 + H_2\)
- \(CH^+_3 + 3 CH_4 \rightarrow (CH_3)_3C^+ + 3H_2\)
Fluoroantimonic acid, HSbF6, can produce solutions with H0 down to –28.[3] Fluoroantimonic acid is made by combining HF and SbF5. In this system, HF releases its proton (H+) concomitant with the binding of F− by antimony pentafluoride, which (as described below) is a Lewis acid. The resulting anion (SbF−6) is both a weak nucleophile and an extraordinarily weak base.
Superacids are useful in reactions such as the isomerization of alkanes. Industrially, anhydrous acid-exchanged zeolites, which are superacid catalysts, are used on a massive scale to isomerize hydrocarbons in the processing of crude oil to gasoline. Superbases such as lithium diethylamide (LiNEt2), alkyllithium compounds (RLi), and Grignard reagents (RMgX) useful in a broad range of organic reactions. LiNEt2 deprotonates C-H bonds to generate reactive carbanions. RLi and RMgX are powerful nucleophiles.
The use of superbases in nonaqueous media allows us to rank the acidities (and measure the pKa's) of different classes of molecules. This ranking is particularly important in understanding the reactions of organic molecules. Note that the order of acidities for hydrocarbons is alkynes >> alkenes, aromatics >> alkanes. This ordering has to do with the hybridization of the carbon atom that forms the carbanion. The negatively charged lone pair of the carbanion is stabilized in orbitals that have high s character (e.g., sp vs. sp2 or sp3). This is because s orbitals have finite probability density at the nucleus and "feel" the positive nuclear charge (thereby stabilizing the extra negative charge on carbon) more than p orbitals. Resonance effects also stabilize carbanions. Thus, cyclopentadiene is more acidic than even an alkyne because the negative charge is delocalized over the entire (aromatic) C5H5- ring when the C5H6 is deprotonated.
| name | formula | structural formula | pKa |
|---|---|---|---|
| Methane | CH4 |  |
~ 56 |
| Propene | C3H6 | 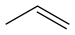 |
~ 44 |
| Benzene | C6H6 | 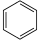 |
~ 43 |
| Acetylene | C2H2 |  |
25 |
| Cyclopentadiene | C5H6 | 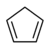 |
18 |

