7.13B: Hard and Soft Metal Centers and Ligands
- Page ID
- 33407
Hard and Soft Acids and Bases, Pearson's HSAB
This theory proposes that soft acids react faster and form stronger bonds with soft bases, whereas hard acids react faster and form stronger bonds with hard bases, all other factors being equal. The classification in the original work was largely based on equilibrium constants for the reaction of two Lewis bases competing for a Lewis acid.
Hard acids and hard bases tend to have the following characteristics:
- small atomic/ionic radius
- high oxidation state
- low polarizability
- high electronegativity (bases)
Examples of hard acids are: H+, light alkali ions (Li through K are considered to have small ionic radii), Ti4+, Cr3+, Cr6+, BF3. Examples of hard bases are: OH-, F-, Cl-, NH3, CH3COO-, CO32-. The affinity of hard acids and hard bases for each other is mainly ionic in nature.
Soft acids and soft bases tend to have the following characteristics:
- large atomic/ionic radius
- low or zero oxidation state bonding
- high polarizability
- low electronegativity
Examples of soft acids are: CH3Hg+, Pt2+, Pd2+, Ag+, Au+, Hg2+, Hg22+, Cd2+, BH3. Examples of soft bases are: H-, R3P, SCN-, I-. The affinity of soft acids and bases for each other is mainly covalent in nature.
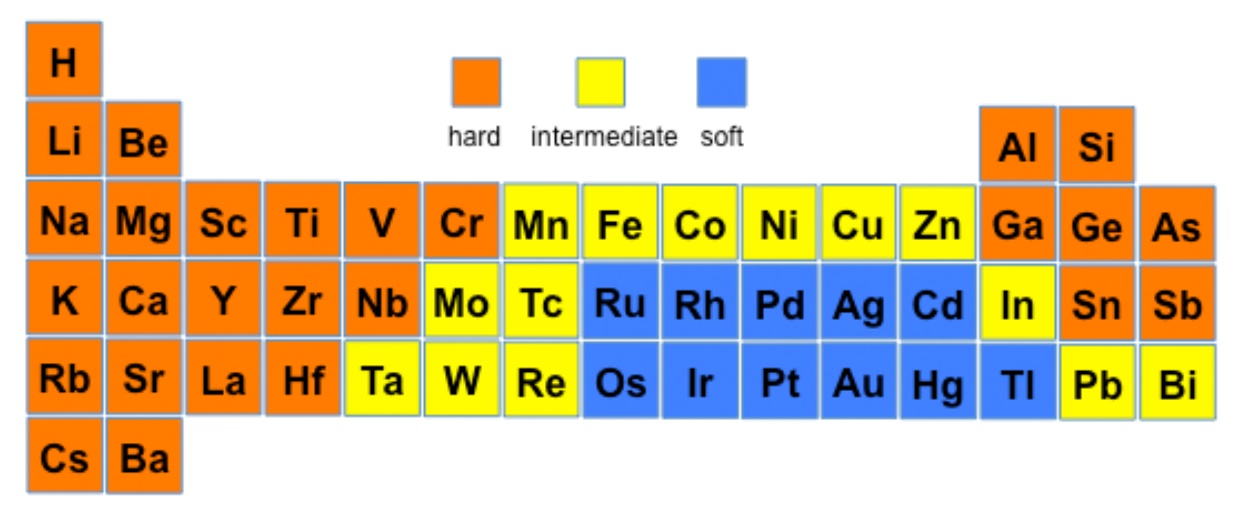
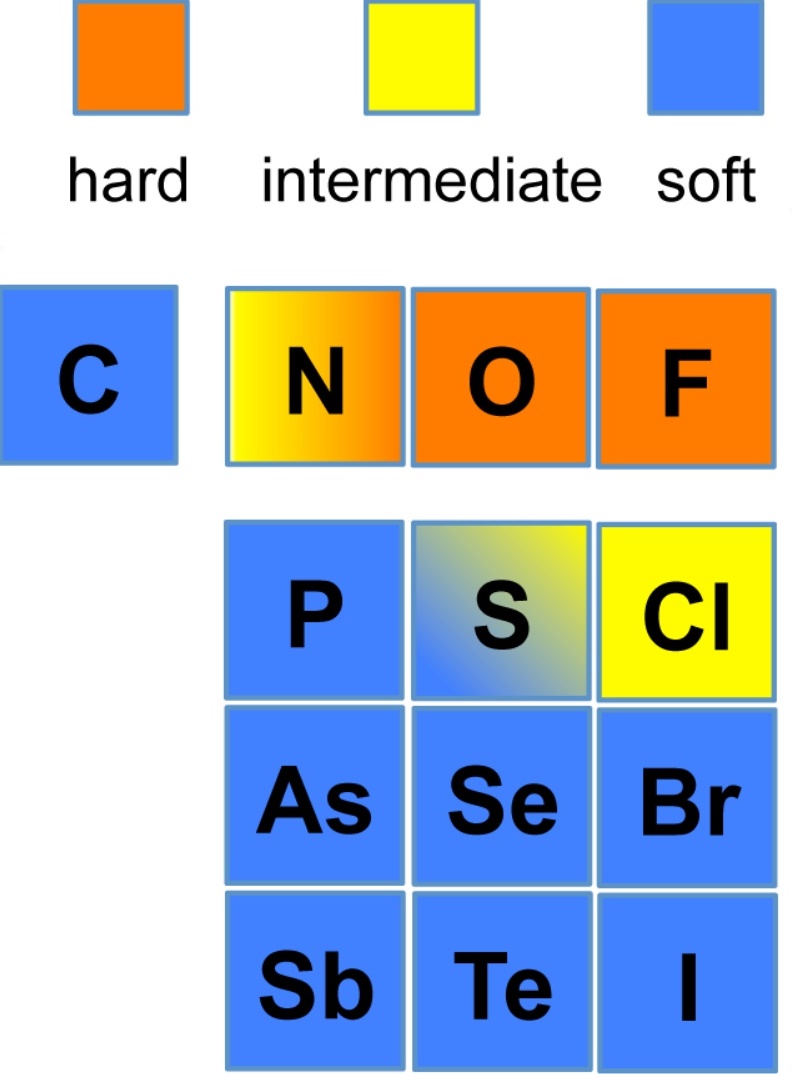
HSAB acids and bases This provides a qualitative approach to looking at the reactions of metal ions with various ligands since, from the diagram above, it is expected that whereas Al(III) and Ti(III) would prefer to react with O-species over S-species, the reverse would be predicted for Hg(II).

