22.1: Periodic Trends in Bonding
- Page ID
- 24335
Periodic trends affect bonding, because of how the elements are arranged on the periodic table. For example elements can be arranged by their electronegative, electron affinity, atomic radius, or ionization energy. Electronegative is the atoms ability to attract other bonded atoms. Electron affinity is an atoms ability to attract another atom. The atomic radius is the radius of an elements atom. Ionization energy is the energy it takes to remove an atom from another atom. Other periodic trends are when the attraction of the atoms for the pair of bonding electrons is different, this is polar covalent bonds. Properties in compounds are used to determine the type of bonding and structure, not just the elements being used. These different properties help group elements to make them either more available or less available for bonding.
Fluorides
It may seem counterintuitive to say that HF is the weakest hydrohalic acid because fluorine has the highest electronegativity. However, the H-F bond is very strong; if the H-X bond is strong, the resulting acid is weak. A strong bond is determined by a short bond length and a large bond dissociation energy. Of all the hydrogen halides, HF has the shortest bond length and largest bond dissociation energy.
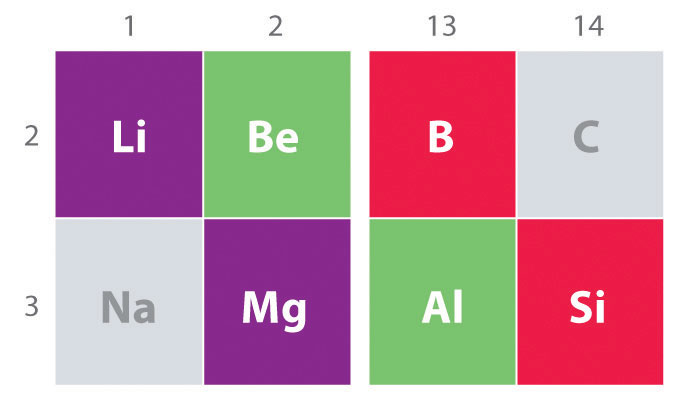
Another important trend to note in main group chemistry is the chemical similarity between the lightest element of one group and the element immediately below and to the right of it in the next group, a phenomenon known as the diagonal effect (Figure \(\PageIndex{1}\) ) There are, for example, significant similarities between the chemistry of Li and Mg, Be and Al, and B and Si. Both BeCl2 and AlCl3 have substantial covalent character, so they are somewhat soluble in nonpolar organic solvents. In contrast, although Mg and Be are in the same group, MgCl2 behaves like a typical ionic halide due to the lower electronegativity and larger size of magnesium.
Oxides
Oxides are binary compounds of oxygen with another element, e.g., CO2, SO2, CaO, CO, ZnO, BaO2, H2O, etc. These are termed as oxides because here, oxygen is in combination with only one element. Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neural:
- An oxide that combines with water to give an acid is termed as an acidic oxide.
- The oxide that gives a base in water is known as a basic oxide.
- An amphoteric solution is a substance that can chemically react as either acid or base.
- However, it is also possible for an oxide to be neither acidic nor basic, but is a neutral oxide.
There are different properties which help distinguish between the three types of oxides. The term anhydride ("without water") refers to compounds that assimilate H2O to form either an acid or a base upon the addition of water.
Acidic Oxides
Acidic oxides are the oxides of non-metals (Groups 14-17) and these acid anhydrides form acids with water:
- Sulfurous Acid
\[ SO_2 + H_2O \rightarrow H_2SO_3 \label{1}\]
- Sulfuric Acid
\[ SO_3 + H_2O \rightarrow H_2SO_4 \label{2}\]
- Carbonic Acid
\[ CO_2 + H_2O \rightarrow H_2CO_3 \label{3}\]
Acidic oxides are known as acid anhydrides (e.g., sulfur dioxide is sulfurous anhydride and sulfur trioxide is sulfuric anhydride) and when combined with bases, they produce salts, e.g.,
\[ SO_2 + 2NaOH \rightarrow Na_2SO_3 + H_2O \label{4}\]
Basic Oxides
Generally Group 1 and Group 2 elements form bases called base anhydrides or basic oxides, e.g.,
\[ K_2O \; (s) + H_2O \; (l) \rightarrow 2KOH \; (aq) \label{5}\]
Basic oxides are the oxides of metals. If soluble in water, they react with water to produce hydroxides (alkalies) e.g.,
\[ CaO + H_2O \rightarrow Ca(OH)_2 \label{6}\]
\[ MgO + H_2O \rightarrow Mg(OH)_2 \label{7}\]
\[ Na_2O + H_2O \rightarrow 2NaOH \label{8}\]
These metallic oxides are known as basic anhydrides. They react with acids to produce salts, e.g.,
\[ MgO + 2HCl \rightarrow MgCl_2 + H_2O \label{9}\]
\[ Na_2O + H_2SO_4 \rightarrow Na_2SO_4 + H_2O \label{10}\]
Amphoteric Oxides
An amphoteric solution is a substance that can chemically react as either acid or base. For example, when HSO4- reacts with water it will make both hydroxide and hydronium ions:
\[ HSO_4^- + H_2O \rightarrow SO_4^{2^-} + H_3O^+ \label{11}\]
\[ HSO_4^- + H_2O \rightarrow H_2SO_4 + OH^- \label{12}\]
Amphoteric oxides are metallic oxides, which show both basic as well as acidic properties. When they react with an acid, they produce salt and water, showing basic properties. While reacting with alkalies they form salt and water showing acidic properties, e.g.,
\[ZnO + 2HCl \rightarrow \underset{\large{zinc\:chloride}}{ZnCl_2}+H_2O\,(basic\: nature) \label{13}\]
\[ZnO + 2NaOH \rightarrow \underset{\large{sodium\:zincate}}{Na_2ZnO_2}+H_2O\,(acidic\: nature) \label{14}\]
\[Al_2O_3 + 3H_2SO_4 \rightarrow Al_2(SO_4)_3+3H_2O\,(basic\: nature) \label{15}\]
\[Al_2O_3 + 2NaOH \rightarrow 2NaAlO_2+H_2O\,(acidic\: nature) \label{16}\]
Amphoteric oxides have both acidic and basic properties. A common example of an amphoteric oxide is aluminum oxide. In general, amphoteric oxides form with metalloids. (see chart below for more detail). Example with acidic properties:
\[ Al_2O_3 + H_2O \rightarrow 2 Al(OH) + 2H^+ \label{17}\]
Example with basic properties:
\[ Al_2O_3 + H_2O \rightarrow 2Al^{3+} + 3OH^- \label{18}\]
Neutral Oxides
Neutral oxides show neither basic nor acidic properties and hence do not form salts when reacted with acids or bases, e.g., carbon monoxide (CO); nitrous oxide (N2O); nitric oxide (NO), etc., are neutral oxides.
Peroxides and Dioxides
Oxides: Group 1 metals react rapidly with oxygen to produce several different ionic oxides, usually in the form of \( M_2O \). With the oyxgen exhibiting an oxidation number of -2.
\[ 4 Li + O_2 \rightarrow 2Li_2O \label{19} \]
Peroxides: Often Lithium and Sodium reacts with excess oxygen to produce the peroxide, \( M_2O_2 \). with the oxidation number of the oxygen equal to -1.
\[ H_2 + O_2 \rightarrow H_2O_2 \label{20}\]
Superoxides: Often Potassium, Rubidium, and Cesium react with excess oxygen to produce the superoxide, \( MO_2 \). with the oxidation number of the oxygen equal to -1/2.
\[ Cs + O_2 \rightarrow CsO_2 \label{21}\]
A peroxide is a metallic oxide which gives hydrogen peroxide by the action of dilute acids. They contain more oxygen than the corresponding basic oxide, e.g., sodium, calcium and barium peroxides.
\[BaO_2 + H_2SO_4 \rightarrow BaSO_4 + H_2O_2 \label{22}\]
\[Na_2O_2 + H_2SO_4 \rightarrow Na_2SO_4 + H_2O_2 \label{23}\]
Dioxides like PbO2 and MnO2 also contain higher percentage of oxygen like peroxides and have similar molecular formulae. These oxides, however, do not give hydrogen peroxide by action with dilute acids. Dioxides on reaction with concentrated HCl yield Cl2 and on reacting with concentrated H2SO4 yield O2.
\[PbO_2 + 4HCl \rightarrow PbCl_2 + Cl_2 + 2H_2O \label{24}\]
\[2PbO_2 + 2H_2SO_4 \rightarrow 2PbSO_4 + 2H_2O + O_2 \label{25}\]
Compound Oxides
Compound oxides are metallic oxides that behave as if they are made up of two oxides, one that has a lower oxidation and one with a higher oxidation of the same metal, e.g.,
\[\textrm{Red lead: } Pb_3O_4 = PbO_2 + 2PbO \label{26}\]
\[\textrm{Ferro-ferric oxide: } Fe_3O_4 = Fe_2O_3 + FeO \label{27}\]
On treatment with an acid, compound oxides give a mixture of salts.
\[\underset{\text{Ferro-ferric oxide}}{Fe_3O_4} + 8HCl \rightarrow \underset{\text{ferric chloride}}{2FeCl_3} + \underset{\text{ferrous chloride}}{FeCl_2} + 4H_2O \label{28}\]
Preparation of Oxides
Oxides can be generated via multiple reactions. Below are a few.
By direct heating of an element with oxygen: Many metals and non-metals burn rapidly when heated in oxygen or air, producing their oxides, e.g.,
\[2Mg + O_2 \xrightarrow{Heat} 2MgO\]
\[2Ca + O_2 \xrightarrow{Heat} 2CaO\]
\[S + O_2 \xrightarrow{Heat} SO_2\]
\[P_4 + 5O_2 \xrightarrow{Heat} 2P_2O_5\]
By reaction of oxygen with compounds at higher temperatures: At higher temperatures, oxygen also reacts with many compounds forming oxides, e.g.,
- sulfides are usually oxidized when heated with oxygen.
\[2PbS + 3O_2 \xrightarrow{\Delta} 2PbO + 2SO_2\]
\[2ZnS + 3O_2 \xrightarrow{\Delta} 2ZnO + 2SO_2\]
- When heated with oxygen, compounds containing carbon and hydrogen are oxidized.
\[C_2H_5OH + 3O_2 \rightarrow 2CO_2 + 3H_2O\]
- By thermal decomposition of certain compounds like hydroxides, carbonates, and nitrates
\[CaCO_3 \xrightarrow{\Delta} CaO + CO_2\]
\[2Cu(NO_3)_2 \xrightarrow{\Delta} 2CuO + 4NO_2 + O_2\]
\[Cu(OH)_2 \xrightarrow{\Delta} CuO + H_2O\]
By oxidation of some metals with nitric acid
\[2Cu + 8HNO_3 \xrightarrow{Heat} 2CuO + 8NO_2 + 4H_2O + O_2\]
\[Sn + 4HNO_3 \xrightarrow{Heat} SnO_2 + 4NO_2 + 2H_2O\]
By oxidation of some non-metals with nitric acid
\[C + 4HNO_3 \rightarrow CO_2 + 4NO_2 + 2H_2O\]
Trends in Acid-Base Behavior
The oxides of elements in a period become progressively more acidic as one goes from left to right in a period of the periodic table. For example, in third period, the behavior of oxides changes as follows:
\[\underset{\large{Basic}}{\underbrace{Na_2O,\: MgO}}\hspace{20px}
\underset{\large{Amphoteric}}{\underbrace{Al_2O_3,\: SiO_2}}\hspace{20px}
\underset{\large{Acidic}}{\underbrace{P_4O_{10},\: SO_3,\:Cl_2O_7}}\hspace{20px}\]
If we take a closer look at a specific period, we may better understand the acid-base properties of oxides. It may also help to examine the physical properties of oxides, but it is not necessary (Table \(\PageIndex{1}\)). Metal oxides on the left side of the periodic table produce basic solutions in water (e.g. Na2O and MgO). Non-metal oxides on the right side of the periodic table produce acidic solutions (e.g. Cl2O, SO2, P4O10). There is a trend within acid-base behavior: basic oxides are present on the left side of the period and acidic oxides are found on the right side.
| 1 | 2 | 3 | 14 | 15 | 16 | 17 |
|
Li |
Be |
B |
C |
N |
O |
F |
|
Na |
Mg |
Al |
Si |
P |
S |
Cl |
|
K |
Ca |
Ga |
Ge |
As |
Se |
Br |
|
Rb |
Sr |
In |
Sn |
Sb |
Te |
I |
|
Cs |
Ba |
Tl |
Pb |
Bi |
Po |
At |
Aluminum oxide shows acid and basic properties of an oxide, it is amphoteric. Thus Al2O3 entails the marking point at which a change over from a basic oxide to acidic oxide occurs. It is important to remember that the trend only applies for oxides in their highest oxidation states. The individual element must be in its highest possible oxidation state because the trend does not follow if all oxidation states are included. Notice how the amphoteric oxides (shown in blue) of each period signify the change from basic to acidic oxides.
Contributors and Attributions
Binod Shrestha (University of Lorraine)

