3.8: Thermochemical Equations
- Page ID
- 49278
Energy changes which accompany chemical reactions are almost always expressed by thermochemical equations, such as
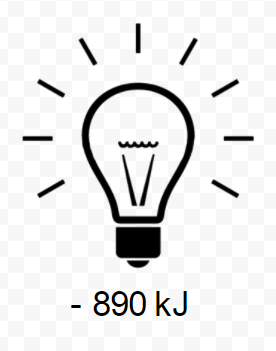
\[\text{C} H_{4} (g) + 2 \text{O}_{2} (g) \rightarrow \text{C} \text{O}_{2} (g) + 2 \text{H}_{2} \text{O} (l) \text{ (25°C, 1 atm pressure)} \\ \Delta H_{m} = –890 \text{kJ} \label{1} \]
which is displayed on the atomic level below. To get an idea of what this reaction looks like on the macroscopic level, check out the flames on the far right.


Here the ΔHm (delta H subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written, and also enables us to find the actual quantity of energy involved. By convention, if ΔHm is positive, heat is absorbed by the reaction; i.e., it is endothermic. More commonly, ΔHm is negative as in Eq. \(\ref{1}\), indicating that heat energy is released rather than absorbed by the reaction, and that the reaction is exothermic. This convention as to whether ΔHm is positive or negative looks at the heat change in terms of the matter actually involved in the reaction rather than its surroundings. In the reaction in Eq. \(\ref{1}\), the C, H, and O atoms have collectively lost energy and it is this loss which is indicated by a negative value of ΔHm.
It is important to notice that ΔHm is the energy for the reaction as written. In the case of Equation \(\ref{1}\), that represents the formation of 1 mol of carbon dioxide and 2 mol of water. The quantity of heat released or absorbed by a reaction is proportional to the amount of each substance consumed or produced by the reaction. Thus Eq. \(\ref{1}\) tells us that 890.4 kJ of heat energy is given off for every mole of CH4 which is consumed. Alternatively, it tells us that 890.4 kJ is released for every 2 moles of H2O produced. Seen in this way, ΔHm is a conversion factor enabling us to calculate the heat absorbed or released when a given amount of substance is consumed or produced. If q is the quantity of heat absorbed or released and n is the amount of substance involved, then
\[\large \Delta H_{\text{m}}=\frac{q}{n} \nonumber \]
How much heat energy is obtained when 1 kg of ethane gas, C2H6, is burned in oxygen according to the equation:
\[2 \text{C}_{2} \text{H}_{6} (g) + 7 \text{O}_{2} (g) \rightarrow 4 \text{C} \text{O}_{2} (g) + 6 \text{H}_{2} \text{O} (l) \nonumber \]
with \(\Delta H_{m} = –3120 \text{ kJ}\).
Solution
The mass of C2H6 is easily converted to the amount of C2H6 from which the heat energy q is easily calculated by means of Eq. (2). The value of ΔHm is –3120 kJ per per 2 mol C2H6. The road map is
\[\large m_{\text{C}_{\text{2}}\text{H}_{\text{6}}}\text{ }\xrightarrow{M}\text{ }n_{\text{C}_{\text{2}}\text{H}_{\text{6}}}\text{ }\xrightarrow{\Delta H_{m}}\text{ }q \nonumber \]
so that\[\begin{align*} q &= 1 \times 10^3 \text{ g }\ce{C2H6} \times \frac{\text{1 mol }\ce{C2H6}}{\text{30.07 g }\ce{C2H6}} \times \frac{-3120\text{ kJ}}{\text{2 mol }\ce{C2H6}} \\ &= -\text{51 879 kJ} = -\text{51.88 MJ} \end{align*} \nonumber \]
By convention, a negative value of q corresponds to a release of heat energy by the matter involved in the reaction.
The quantity ΔHm is referred to as an enthalpy change for the reaction. In this context the symbol Δ (delta) signifies change in” while H is the symbol for the quantity being changed, namely the enthalpy. We will deal with the enthalpy in some detail in Chap. 15. For the moment we can think of it as a property of matter which increases when matter absorbs energy and decreases when matter releases energy.
It is important to realize that the value of ΔHm given in thermochemical equations like \(\ref{1}\) or \(\ref{3}\) depends on the physical state of both the reactants and the products. Thus, if water were obtained as a gas instead of a liquid in the reaction in Eq. \(\ref{1}\), the value of ΔHm would be different from -890.4 kJ. It is also necessary to specify both the temperature and pressure since the value of ΔHm depends very slightly on these variables. If these are not specified they usually refer to 25°C and to normal atmospheric pressure.
Two more characteristics of thermochemical equations arise from the law of conservation of energy. The first is that writing an equation in the reverse direction changes the sign of the enthalpy change. For example,
\[ \text{H}_{2} \text{O} (l) \rightarrow \text{H}_{2} \text{O} (g) \\ \Delta \text{H}_{m} = 44 \text{ kJ} \nonumber \]

In the image above, the flames input energy into the water, giving it the energy necessary to transition to the gas phase. Since flames provide the energy for the phase transition, this is an endothermic reaction (energy is absorbed).
tells us that when a mole of liquid water vaporizes, 44 kJ of heat is absorbed. This corresponds to the fact that heat is absorbed from your skin when perspiration evaporates, and you cool off. Condensation of 1 mol of water vapor, on the other hand, gives off exactly the same quantity of heat. \[ \text{H}_{2} \text{O} (g) \rightarrow \text{H}_{2} \text{O} (l) \\ \Delta \text{H}_{m} = –44 \text{kJ} \nonumber \]

It's counterintuitive, but the common summer occurrence seen above is actually exothermic. Since the reaction isn't highly exothermic (like the combustion of CH4), we find it hard to associate with a release of energy. Thermodynamics allows us to better understand on a micro level energy changes like this one.
To see why this must be true, suppose that ΔHm [Eq. (4a)] = 44 kJ while ΔHm [Eq. (4b)] = –50.0 kJ. If we took 1 mol of liquid water and allowed it to evaporate, 44 kJ would be absorbed. We could then condense the water vapor, and 50.0 kJ would be given off. We could again have 1 mol of liquid water at 25°C but we would also have 6 kJ of heat which had been created from nowhere! This would violate the law of conservation of energy. The only way the problem can he avoided is for ΔHm of the reverse reaction to be equal in magnitude but opposite in sign from ΔHm of the forward reaction. That is,
\[ \Delta \text{H}_{m} \text{forward} = –\Delta \text{H}_{m} \text{reverse} \nonumber \]


