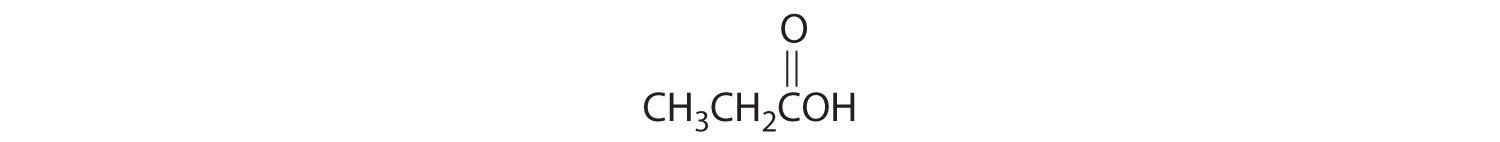3.E: Homework Problems
- Page ID
- 13793
3.1: Types of Chemical Compounds and their Formulas
Conceptual Problems
1. Ionic and covalent compounds are held together by electrostatic attractions between oppositely charged particles. Describe the differences in the nature of the attractions in ionic and covalent compounds. Which class of compounds contains pairs of electrons shared between bonded atoms?
2. Which contains fewer electrons than the neutral atom—the corresponding cation or the anion?
3. What is the difference between an organic compound and an inorganic compound?
4. What is the advantage of writing a structural formula as a condensed formula?
5. The majority of elements that exist as diatomic molecules are found in one group of the periodic table. Identify the group.
6. Discuss the differences between covalent and ionic compounds with regard to
a. the forces that hold the atoms together.
b. melting points.
c. physical states at room temperature and pressure.
7. Why do covalent compounds generally tend to have lower melting points than ionic compounds?
Answer 7. Covalent compounds generally melt at lower temperatures than ionic compounds because the intermolecular interactions that hold the molecules together in a molecular solid are weaker than the electrostatic attractions that hold oppositely charged ions together in an ionic solid.
Numerical Problems
1. The structural formula for chloroform (CHCl3) was shown in Example 2. Based on this information, draw the structural formula of dichloromethane (CH2Cl2).
2. What is the total number of electrons present in each ion?
a. F−
b. Rb+
c. Ce3+
d. Zr4+
e. Zn2+
f. Kr2+
g. B3+
3. What is the total number of electrons present in each ion?
a. Ca2+
b. Se2−
c. In3+
d. Sr2+
e. As3+
f. N3−
g. Tl+
4. Predict how many electrons are in each ion.
a. an oxygen ion with a −2 charge
b. a beryllium ion with a +2 charge
c. a silver ion with a +1 charge
d. a selenium ion with a +4 charge
e. an iron ion with a +2 charge
f. a chlorine ion with a −1 charge
5. Predict how many electrons are in each ion.
a. a copper ion with a +2 charge
b. a molybdenum ion with a +4 charge
c. an iodine ion with a −1 charge
d. a gallium ion with a +3 charge
e. an ytterbium ion with a +3 charge
f. a scandium ion with a +3 charge
6. Predict the charge on the most common monatomic ion formed by each element.
a. chlorine
b. phosphorus
c. scandium
d. magnesium
e. arsenic
f. oxygen
7. Predict the charge on the most common monatomic ion formed by each element.
a. sodium
b. selenium
c. barium
d. rubidium
e. nitrogen
f. aluminum
8. For each representation of a monatomic ion, identify the parent atom, write the formula of the ion using an appropriate superscript, and indicate the period and group of the periodic table in which the element is found.
a. \(_4^9X^{2+} \)
b. \(_1^1X^-\)
c. \(_8^{16}X^{2-} \)
9. For each representation of a monatomic ion, identify the parent atom, write the formula of the ion using an appropriate superscript, and indicate the period and group of the periodic table in which the element is found.
a. \(_3^7X^+ \)
b. \(_9^{19}X^-\)
c. \(_{13}^{27}X^{3+}\)
Answers
5.
a. 27
b. 38
c. 54
d. 28
e. 67
f. 18
9.
a. Li, Li+, 2nd period, group 1
b. F, F–, 2nd period, group 17
c. Al, Al3+, 3nd period, group 13
Conceptual Problems2
1. What are the differences and similarities between a polyatomic ion and a molecule?
2. Classify each compound as ionic or covalent.
- Zn3(PO4)2
- C6H5CO2H
- K2Cr2O7
- CH3CH2SH
- NH4Br
- CCl2F2
3. Classify each compound as ionic or covalent. Which are organic compounds and which are inorganic compounds?
- CH3CH2CO2H
- CaCl2
- Y(NO3)3
- H2S
- NaC2H3O2
4. Generally, one cannot determine the molecular formula directly from an empirical formula. What other information is needed?
5. Give two pieces of information that we obtain from a structural formula that we cannot obtain from an empirical formula.
6. The formulas of alcohols are often written as ROH rather than as empirical formulas. For example, methanol is generally written as CH3OH rather than CH4O. Explain why the ROH notation is preferred.
7. The compound dimethyl sulfide has the empirical formula C2H6S and the structural formula CH3SCH3. What information do we obtain from the structural formula that we do not get from the empirical formula? Write the condensed structural formula for the compound.
8. What is the correct formula for magnesium hydroxide—MgOH2 or Mg(OH)2? Why?
9. Magnesium cyanide is written as Mg(CN)2, not MgCN2. Why?
10. Does a given hydrate always contain the same number of waters of hydration?
Answers 2
7. The structural formula gives us the connectivity of the atoms in the molecule or ion, as well as a schematic representation of their arrangement in space. Empirical formulas tell us only the ratios of the atoms present. The condensed structural formula of dimethylsulfide is (CH3)2S.
Numerical Problems 2
1. Write the formula for each compound.
a. magnesium sulfate, which has 1 magnesium atom, 4 oxygen atoms, and 1 sulfur atom
b. ethylene glycol (antifreeze), which has 6 hydrogen atoms, 2 carbon atoms, and 2 oxygen atoms
c. acetic acid, which has 2 oxygen atoms, 2 carbon atoms, and 4 hydrogen atoms
d. potassium chlorate, which has 1 chlorine atom, 1 potassium atom, and 3 oxygen atoms
e. sodium hypochlorite pentahydrate, which has 1 chlorine atom, 1 sodium atom, 6 oxygen atoms, and 10 hydrogen atoms
2. Write the formula for each compound.
a. cadmium acetate, which has 1 cadmium atom, 4 oxygen atoms, 4 carbon atoms, and 6 hydrogen atoms
b. barium cyanide, which has 1 barium atom, 2 carbon atoms, and 2 nitrogen atoms
c. iron(III) phosphate dihydrate, which has 1 iron atom, 1 phosphorus atom, 6 oxygen atoms, and 4 hydrogen atoms
d. manganese(II) nitrate hexahydrate, which has 1 manganese atom, 12 hydrogen atoms, 12 oxygen atoms, and 2 nitrogen atoms
e. silver phosphate, which has 1 phosphorus atom, 3 silver atoms, and 4 oxygen atoms
3. Complete the following table by filling in the formula for the ionic compound formed by each cation-anion pair.
| Ion | K+ | Fe3+ | NH4+ | Ba2+ |
|---|---|---|---|---|
| Cl− | KCl | |||
| SO42− | ||||
| PO43− | ||||
| NO3− | ||||
| OH− |
4. Write the empirical formula for the binary compound formed by the most common monatomic ions formed by each pair of elements.
a. zinc and sulfur
b. barium and iodine
c. magnesium and chlorine
d. silicon and oxygen
e. sodium and sulfur
5. Write the empirical formula for the binary compound formed by the most common monatomic ions formed by each pair of elements.
a. lithium and nitrogen
b. cesium and chlorine
c. germanium and oxygen
d. rubidium and sulfur
e. arsenic and sodium
6. Write the empirical formula for each compound.
a. Na2S2O4
b. B2H6
c. C6H12O6
d. P4O10
e. KMnO4
7. Write the empirical formula for each compound.
a. Al2Cl6
b. K2Cr2O7
c. C2H4
d. (NH2)2CNH
e. CH3COOH
Answers
1.
a. MgSO4
b. C2H6O2
c. C2H4O2
d. KClO3
e. NaOCl·5H2O
3.
| Ion | K + | Fe 3+ | NH 4 + | Ba 2+ |
|---|---|---|---|---|
| Cl − | KCl | FeCl3 | NH4Cl | BaCl2 |
| SO 4 2− | K2SO4 | Fe2(SO4)3 | (NH4)2SO4 | BaSO4 |
| PO 4 3− | K3PO4 | FePO4 | (NH4)3PO4 | Ba3(PO4)2 |
| NO 3 − | KNO3 | Fe(NO3)3 | NH4NO3 | Ba(NO3)2 |
| OH − | KOH | Fe(OH)3 | NH4OH | Ba(OH)2 |
5.
a. Li3N
b. CsCl
c. GeO2
d. Rb2S
e. Na3As
7.
a. AlCl3
b. K2Cr2O7
c. CH2
d. CH5N3
e. CH2O
3.6: Names and Formulas of Inorganic Compounds
Conceptual Problems
1. Name each cation.
- a. K+
- b. Al3+
- c. NH4+
- d. Mg2+
- e. Li+
2. Name each anion.
- a. Br−
- b. CO32−
- c. S2−
- d. NO3−
- e. HCO2−
- f. F−
- g. ClO−
- h. C2O42−
3. Name each anion.
- a. PO43−
- b. Cl−
- c. SO32−
- d. CH3CO2−
- e. HSO4−
- f. ClO4−
- g. NO2−
- h. O2−
4. Name each anion.
- a. SO42−
- b. CN−
- c. Cr2O72−
- d. N3−
- e. OH−
- f. I−
- g. O22−
5. Name each compound.
- a. MgBr2
- b. NH4CN
- c. CaO
- d. KClO3
- e. K3PO4
- f. NH4NO2
- g. NaN3
6. Name each compound.
- a. NaNO3
- b. Cu3(PO4)2
- c. NaOH
- d. Li4C
- e. CaF2
- f. NH4Br
- g. MgCO3
7. Name each compound.
- a. RbBr
- b. Mn2(SO4)3
- c. NaClO
- d. (NH4)2SO4
- e. NaBr
- f. KIO3
- g. Na2CrO4
8. Name each compound.
- a. NH4ClO4
- b. SnCl4
- c. Fe(OH)2
- d. Na2O
- e. MgCl2
- f. K2SO4
- g. RaCl2
9. Name each compound.
- a. KCN
- b. LiOH
- c. CaCl2
- d. NiSO4
- e. NH4ClO2
- f. LiClO4
- g. La(CN)3
Answer
7.
a. rubidium bromide
b. manganese(III) sulfate
c. sodium hypochlorite
d. ammonium sulfate
e. sodium bromide
f. potassium iodate
g. sodium chromate
Numerical Problems
1. For each ionic compound, name the cation and the anion and give the charge on each ion.
- a. BeO
- b. Pb(OH)2
- c. BaS
- d. Na2Cr2O7
- e. ZnSO4
- f. KClO
- g. NaH2PO4
2. For each ionic compound, name the cation and the anion and give the charge on each ion.
- a. Zn(NO3)2
- b. CoS
- c. BeCO3
- d. Na2SO4
- e. K2C2O4
- f. NaCN
- g. FeCl2
3. Write the formula for each compound.
- a. magnesium carbonate
- b. aluminum sulfate
- c. potassium phosphate
- d. lead(IV) oxide
- e. silicon nitride
- f. sodium hypochlorite
- g. titanium(IV) chloride
- h. disodium ammonium phosphate
4. Write the formula for each compound.
- a. lead(II) nitrate
- b. ammonium phosphate
- c. silver sulfide
- d. barium sulfate
- e. cesium iodide
- f. sodium bicarbonate
- g. potassium dichromate
- h. sodium hypochlorite
5. Write the formula for each compound.
- a. zinc cyanide
- b. silver chromate
- c. lead(II) iodide
- d. benzene
- e. copper(II) perchlorate
6. Write the formula for each compound.
- a. calcium fluoride
- b. sodium nitrate
- c. iron(III) oxide
- d. copper(II) acetate
- e. sodium nitrite
7. Write the formula for each compound.
- a. sodium hydroxide
- d. calcium cyanide
- c. magnesium phosphate
- d. sodium sulfate
- e. nickel(II) bromide
- f. calcium chlorite
- g. titanium(IV) bromide
8. Write the formula for each compound.
- a. sodium chlorite
- b. potassium nitrite
- c. sodium nitride (also called sodium azide)
- d. calcium phosphide
- e. tin(II) chloride
- f. calcium hydrogen phosphate
- g. iron(II) chloride dihydrate
9. Write the formula for each compound.
- a. potassium carbonate
- b. chromium(III) sulfite
- c. cobalt(II) phosphate
- d. magnesium hypochlorite
- e. nickel(II) nitrate hexahydrate
Conceptual Problems
1. Name each acid.
- a. HCl
- b. HBrO3
- c. HNO3
- d. H2SO4
- e. HIO3
2. Name each acid.
- a. HBr
- b. H2SO3
- c. HClO3
- d. HCN
- e. H3PO4
3. Name the aqueous acid that corresponds to each gaseous species.
- a. hydrogen bromide
- b. hydrogen cyanide
- c. hydrogen iodide
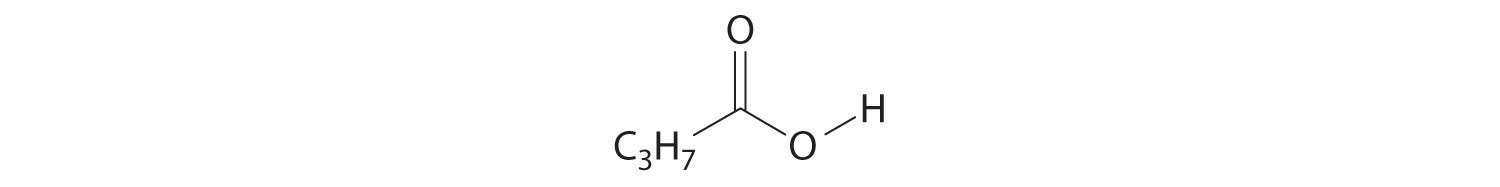
4. For each structural formula, write the condensed formula and the name of the compound.
a.
b.
5. For each structural formula, write the condensed formula and the name of the compound.
a.
b.
6. When each compound is added to water, is the resulting solution acidic, neutral, or basic?
- a. CH3CH2OH
- b. Mg(OH)2
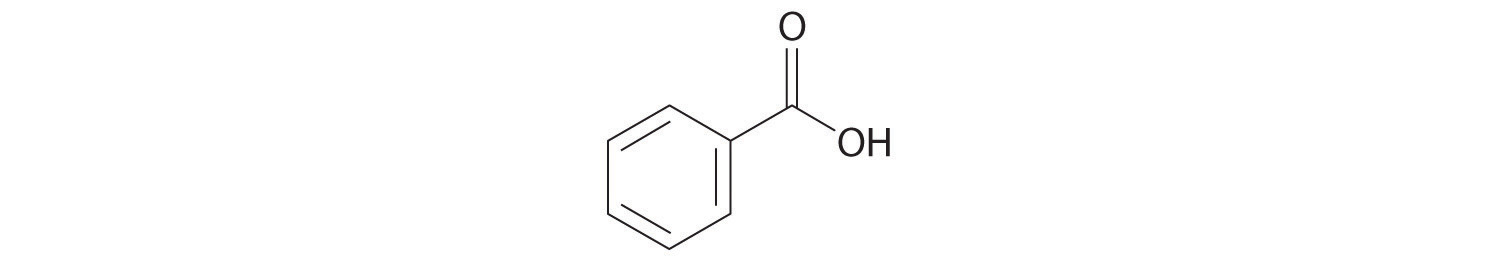
- c. C6H5CO2H
- d. LiOH
- e. C3H7CO2H
- f. H2SO4
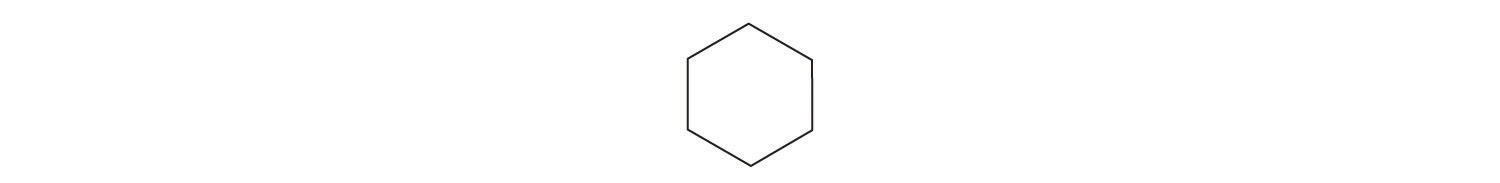
7. Draw the structure of the simplest example of each type of compound.
- a. alkane
- b. alkene
- c. alkyne
- d. aromatic hydrocarbon
- e. alcohol
- f. carboxylic acid
- g. amine
- h. cycloalkane
8. Identify the class of organic compound represented by each compound.
a.
b. CH3CH2OH
c. HC≡CH
d.
e. C3H7NH2
f. CH3CH=CHCH2CH3
g.
h.
9. Identify the class of organic compound represented by each compound.
a.
b.
c.
d.
e.
f. CH3C≡CH
g.
h.
Numerical Problems
1. Write the formula for each compound.
- a. hypochlorous acid
- b. perbromic acid
- c. hydrobromic acid
- d. sulfurous acid
- e. sodium perbromate
2. Write the formula for each compound.
- a. hydroiodic acid
- b. hydrogen sulfide
- c phosphorous acid
- d. perchloric acid
- e. calcium hypobromite
3. Name each compound.
- a. HBr
- b. H2SO3
- c. HCN
- d. HClO4
- e. NaHSO4
4. Name each compound.
- a. H2SO4
- b. HNO2
- c. K2HPO4
- d. H3PO3
- e. Ca(H2PO4)2·H2O