22.7: Corrosion
- Page ID
- 49651
An important aspect of the use of some metals, particularly of iron, is the possibility of corrosion. It is estimated that about one-seventh of all iron production goes to replace the metal lost to corrosion. Rust is apparently a hydrated form of iron(III)oxide. The formula is approximately Fe2O3•\(\tfrac{\text{3}}{\text{2}}\)H2O, although the exact amount of water is variable. (Note that this is about halfway between iron(III) hydroxide, Fe(OH)3 or ½{Fe2O3•3H2O], and anhydrous Fe2O3).
Rusting requires both oxygen and water, and it is usually sped up by acids, strains in the iron, contact with less-active metals, and the presence of rust itself. In addition, observation of a rusted object, such as an iron nail from an old wooden building, shows that rust will deposit in one location (near the head of the nail) while the greatest loss of metallic iron will occur elsewhere (near the point). These facts suggest that the mechanism of rusting involves a galvanic cell. The half-equations involved are
\[\text{2Fe}(S) \rightarrow \text{2Fe}^{2+}(aq) + \text{4}e^-\label{1} \]
\[\text{4}e^- + \text{4H}^+(aq) + \text{O}_2(g) \rightarrow \text{2H}_2\text{O}\label{2} \]
yielding the full reaction:
\[\text{2Fe}(s) + \text{4H}^+(aq) + \text{O}_2(g) \rightarrow \text{2Fe}^{2+}(aq) + \text{2H}_2\text{O}\label{3} \]
Once Fe2+(aq) is formed, it can migrate freely through the aqueous solution to another location on the metal surface. At that point the iron can precipitate:
\[\text{4Fe}(s) + \text{O}_2(g) + \text{7 H}_2\text{O}(l) \rightarrow \text{2Fe}_2\text{O}_3 \cdot \frac{3}{2} \text{H}_2\text{O}(s) + \text{8H}^+(aq) \nonumber \]
Hydrogen ions liberated by this reaction are then partially consumed by Equation \(\ref{2}\). The electrons required for half-equation \(\ref{2}\) are supplied from Equation \(\ref{1}\) via metallic conduction through the iron or by ionic conduction if the aqueous solution contains a significant concentration of ions. Thus iron rusts faster in contact with salt water than in fresh.
The mechanism proposed in the preceding paragraph implies that some regions of the iron surface become cathodic, i.e., that reduction of oxygen to water occurs there. Other locations are anodic; oxidation of Fe to Fe2+ occurs. The chief way in which such regions may be set up depends on restriction of oxygen supply, because oxygen is required for the cathodic reaction shown in Equation \(\ref{2}\). In the case of the iron nail, for example, rust forms near the head because more oxygen is available. Most of the loss of metal takes place deep in the wood, however, near the point of the nail. At this location Equation \(\ref{1}\) but not \(\ref{2}\) can occur.
A similar situation occurs when a drop of moisture adheres to an iron surface (Figure \(\PageIndex{1}\)). Pitting occurs near the center of the drop, while hydrated iron(III) oxide deposits near the edge.
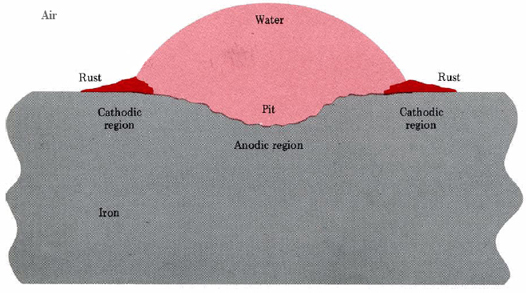
A second way in which anodic and cathodic regions may be set up involves the presence of a second metal which has a greater attraction for electrons (is less easily oxidized) than iron. Such a metal can drain off electrons left behind in the iron when Fe2+ dissolves. This excess of electrons makes the less-active metal an ideal site for Equation \(\ref{2}\), and so a cell is set up at the intersection of the metals. Rust may actually coat the surface of the less-active metal while pits form in the iron.
The most important technique for rust prevention is simply to exclude water and oxygen by means of a protective coating. This is the principle behind oiling, greasing, painting, or metal plating of iron. The coating must be complete, however, or rusting may be accelerated by exclusion of oxygen from part of the surface. This is especially true when iron is coated with a less-active metal such as tin. Even a pinhole in the coating on a tin can will rust very quickly, since the tin becomes cathodic due to its larger electrode potential and to the oxygen exclusion from the iron beneath.
A second technique involves bringing the iron object in contact with a more active metal. This is called cathodic protection because the more active metal donates electrons to the iron, strongly inhibiting Equation \(\ref{1}\). Both cathodic protection and a surface coating are provided by galvanizing, a process in which zinc is plated onto steel electrolytically or by dipping in the molten metal. Like many other metals, zinc is self-protective—it reacts with oxygen and carbon dioxide from air to form an adherent impervious coating of zinc hydroxycarbonate, Zn2(OH)2CO3. Should there be a scratch in the zinc plate, the iron still cannot rust because zinc will be preferentially oxidized. The hydroxycarbonate formed will then cover the opening, preventing further contact of oxygen with the iron or zinc.
A third technique applies to situations (such as an automobile radiator) where aqueous solutions are in contact with the iron. Corrosion inhibitors include chromate salts and organic compounds such as tributylamine, (C4H9)3N. Chromates apparently form an impervious coating of FeCrO4(s) as soon as any iron is oxidized to iron(II). Tributylamine, a derivative of ammonia, reacts with organic acids formed by decomposition of antifreeze at the high temperatures of an automobile engine. The tributylammonium salts produced are insoluble and coat the inside of the cooling system. Thus tributylamine neutralizes acid which would accelerate corrosion and provides a surface coating as well.


