20.6: Polar Lipids
- Page ID
- 49607
As was true of most nonpolar lipids, the structures of polar lipids are based on condensation of fatty acids with glycerol. The main difference is that only two of the three OH groups on glycerol are involved. The third is combined with a highly polar molecule:
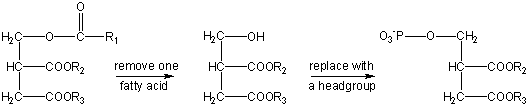
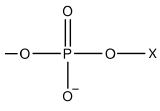 where X can be a number of functional groups, such as choline, glycerol, ethanolamine, and serine, the example we have given. This give a structure where there is a highly polar head group, with two longer, non-polar fatty acid chain tails. This structure is often generalized in a cartoon form also shown in Figure \(\PageIndex{1}\).
where X can be a number of functional groups, such as choline, glycerol, ethanolamine, and serine, the example we have given. This give a structure where there is a highly polar head group, with two longer, non-polar fatty acid chain tails. This structure is often generalized in a cartoon form also shown in Figure \(\PageIndex{1}\).In one sense the polar lipids are like the anions of fatty acids, only more so. They contain two hydrophobic hydrocarbon tails and a head which may have several electrically charged sites. As in the case of soap and detergent molecules, the tails of polar lipids tend to avoid water and other polar substances, but the heads are quite compatible with such environments.
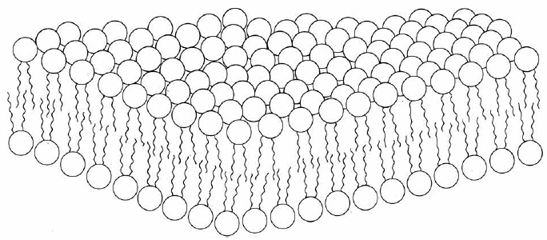
The polar lipids are most commonly found as components of cell walls and other membranes. Nearly all hypotheses regarding membrane structure take as a fundamental component a lipid bilayer (Figure \(\PageIndex{2}\) ). Bilayers made in the laboratory have many properties in common with membranes. Ions such as Na+, K+, and Cl– cannot pass through them, but water molecules can. The hydrocarbon core of such a bilayer should have large electrical resistance, as does a membrane. Certain carrier molecules can transport K+ and other ions across a bilayer, apparently by wrapping a hydrophobic cloak around them to disguise their charges. Membrane proteins in a bilayer also allow for transport of ions and other molecules across the bilayer which could not cross otherwise.


