8.24: Addition Polymers
- Page ID
- 49486
Addition polymers are usually made from a monomer containing a double bond. We can think of the double bond as "opening out" in order to participate in two new single bonds in the following way:
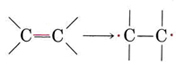
Thus, if ethene is heated at moderate temperature and pressure in the presence of an appropriate catalyst, it polymerizes:

| Monomer | Nonsystematic Name | Polymer | Some Typical Uses |
|---|---|---|---|
| Ethylene | Polyethylene | Film for packaging and bags, toys, bottles, coatings | |
| Propylene | Polypropylene | Milk cartons, rope, outdoor carpeting | |
| Styrene | Polystyrene | Transparent containers, plastic glasses, refrigerators, styrofoam | |
| Vinyl chloride | Polyvinyl chloride, PVC | Pipe and tubing, raincoats, curtains, phonograph records, luggage, floor tiles | |
| Acrylonitrile | Polyacrylonitrile (Orlon, Acrilan) | Textiles, ruga | |
| Tetrafluoroethylene | Teflon | Nonstick pan coatings, bearings, gaskets |
The result is the familiar waxy plastic called polyethylene, which at a molecular level consists of a collection of long-chain alkane molecules, most of which contain tens of thousands of carbon atoms. There is only an occasional short branch chain.
Polyethylene is currently manufactured on a very large scale, larger than any other polymer, and is used for making plastic bags, cheap bottles, toys, etc. Many of its properties are what we would expect from its molecular composition. The fact that it is a mixture of molecules each of slightly different chain length (and hence slightly different melting point) explains why it softens over a range of temperatures rather than having a single melting point. Because the molecules are only held together by London forces, this melting and softening occurs at a rather low temperature. (Some of the cheaper varieties of polyethylene with shorter chains and more branch chains will even soften in boiling water.) The same weak London forces explain why polyethylene is soft and easy to scratch and why it is not very ‘strong mechanically.'
The table above lists some other well-known addition polymers and also some of their uses. You can probably find at least one example of each of them in your home. Except for Teflon, all these polymers derive from a monomer of the form.
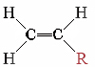
The resulting polymer thus has the general form:
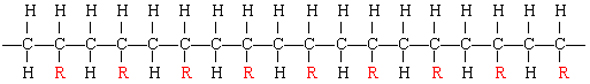
By varying the nature of the R group, the physical properties of the polymer can be controlled rather precisely.


