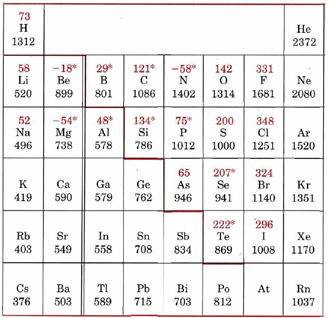
8.4.5: Ionization Energies and Electron Affinities
- Page ID
- 49765
* Electron affinities marked with an asterisk (*) have been obtained from theoretical calculations rather than experimental measurements. The heavy colored line separates metals (ionization energy usually below about 800 kJ mol–1) from nonmetals.
This table gives ionization energies and electron affinities for common elements, and displays the information in terms of the periodic table. Ionization energies are in black, with electron affinities in red. For ionization energies, two general tendencies arise. First, as one moves down a given group in the periodic table, the ionization energy decreases. Second, as one moves from left to right across the periodic table (from an alkali-metal atom to a noble gas), the ionization energy increases on the whole. While electron affinities display fewer regularities on the table, trends do exist. All the halogens have values of about 300 kJ mol–1 while the group VI nonmetals have somewhat lower values, in the region of 200 kJ mol–1 or less.