8.10.9A: Electrolytes and Electrolytic Solutions
- Page ID
- 20109
Make sure you thoroughly understand the following essential ideas:
- Describe the properties of water that make it an ideal electrolytic solvent.
- Describe the general structure of ionic hydration shells.
- Explain why all cations act as acids in water.
- Describe some of the major ways in which the conduction of electricity through a solution differs from metallic conduction.
- Define resistance, resistivity, conductance, and conductivity.
- Define molar conductivity and explain its significance.
- Explain the major factors that cause molar conductivity to diminish as electrolyte concentrations increase.
- Describe the contrasting behavior of strong, intermediate, and weak electrolytes.
- Explain the distinction between ionic diffusion and ionic migration.
- Define the limiting ionic conductivity, and comment on some of its uses.
- Explain why hydrogen- and hydroxide ions exhibit exceptionally large ionic mobilities.
Electrolytic solutions are those that are capable of conducting an electric current. A substance that, when added to water, renders it conductive, is known as an electrolyte. A common example of an electrolyte is ordinary salt, sodium chloride. Solid NaCl and pure water are both non-conductive, but a solution of salt in water is readily conductive. A solution of sugar in water, by contrast, is incapable of conducting a current; sugar is therefore a non-electrolyte.
These facts have been known since 1800 when it was discovered that an electric current can decompose the water in an electrolytic solution into its elements (a process known as electrolysis). By mid-century, Michael Faraday had made the first systematic study of electrolytic solutions. Faraday recognized that for a sample of matter to conduct electricity, two requirements must be met:
- The matter must be composed of, or contain, electrically charged particles.
- These particles must be mobile; that is, they must be free to move under the influence of an external applied electric field.
In metallic solids, the charge carriers are electrons rather than ions; their mobility is a consequence of the quantum-mechanical uncertainty principle which promotes the escape of the electrons from the confines of their local atomic environment. In the case of electrolytic solutions, Faraday called the charge carrier ions (after the Greek word for "wanderer"). His most important finding was that each kind of ion (which he regarded as an electrically-charged atom) carries a definite amount of charge, most commonly in the range of ±1-3 units.
The fact that the smallest charges observed had magnitudes of ±1 unit suggested an "atomic" nature for electricity itself, and led in 1891 to the concept of the "electron" as the unit of electric charge — although the identification of this unit charge with the particle we now know as the electron was not made until 1897.
An ionic solid such as NaCl is composed of charged particles, but these are held so tightly in the crystal lattice that they are unable to move about, so the second requirement mentioned above is not met and solid salt is not a conductor. If the salt is melted or dissolved in water, the ions can move freely and the molten liquid or the solution becomes a conductor.
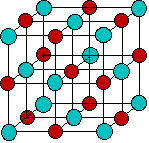
Since positively-charged ions are attracted to a negative electrode that is traditionally known as the cathode, these are often referred to as cations. Similarly, negatively-charged ions, being attracted to the positive electrode, or anode, are called anions. (These terms were all coined by Faraday.)
The role of the solvent: what's special about water
Although we tend to think of the solvent (usually water) as a purely passive medium within which ions drift around, it is important to understand that electrolytic solutions would not exist without the active involvement of the solvent in reducing the strong attractive forces that hold solid salts and molecules such as HCl together. Once the ions are released, they are stabilized by interactions with the solvent molecules. Water is not the only liquid capable of forming electrolytic solutions, but it is by far the most important. It is therefore essential to understand those properties of water that influence the stability of ions in aqueous solution.
According to Coulomb's law, the force between two charged particles is directly proportional to the product of the two charges, and inversely proportional to the square of the distance between them:
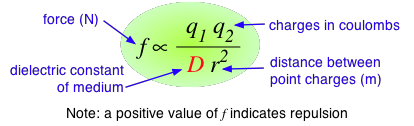
The proportionality constant D is the dimensionless dielectric constant. Its value in empty space is unity, but in other media it will be larger. Since D appears in the denominator, this means that the force between two charged particles within a gas or liquid will be less than if the particles were in a vacuum. Water has one of the highest dielectric constants of any known liquid; the exact value varies with the temperature, but 80 is a good round number to remember. When two oppositely-charged ions are immersed in water, the force acting between them is only 1/80 as great as it would be between the two gaseous ions at the same distance. It can be shown that in order to separate one mole of Na+ and Cl– ions at their normal distance of 23.6 pm in solid sodium chloride, the work required will be 586 J in a vacuum, but only 7.3 J in water
Dielectric Constant
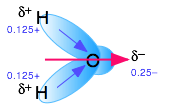
The dielectric constant is a bulk property of matter, rather than being a property of the molecule itself, as is the dipole moment. It is a cooperative effect of all the molecules in the liquid, and is a measure of the extent to which an applied electric field will cause the molecules to line up with the negative ends of their dipoles pointing toward the positive direction of the electric field. The high dielectric constant of water is a consequence of the small size of the H2O molecule in relation to its large dipole moment.
When one molecule is reoriented by the action of an external electric field, local hydrogen bonding tends to pull neighboring molecules into the same alignment, thus producing an additive effect that would be absent if the molecules were all acting independently.
Water's dipole moment stabilizes dissolved ions through hydrogen bonding
When an ion is introduced into a solvent, the attractive interactions between the solvent molecules must be disrupted to create space for the ion. This costs energy and would by itself tend to inhibit dissolution. However, if the solvent has a high permanent dipole moment, the energy cost is more than recouped by the ion-dipole attractions between the ion and the surrounding solvent molecules.
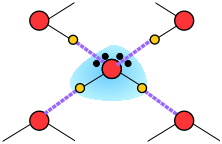
Water, as you know, has a sizeable dipole moment that is the source of the hydrogen bonding that holds the liquid together. The greater strength of ion-dipole attractions compared to hydrogen-bonding (dipole-dipole) attractions stabilizes the dissolved ion.
Water is not the only electrolytic solvent, but it is by far the best. For some purposes, chemists occasionally need to employ non-aqueous solvents when studying electrolytes. Here are a few examples:
| solvent | Melting Point (°C) | Boiling Point °C | D | dipole moment | specific conductivity, S/cm |
|---|---|---|---|---|---|
| water | 0 | 100 | 80.1 | 1.87 | 5.5 × 10–8 |
| methanol | –98 | 64.7 | 32.7 | 1.7 | 1.5× 10–7 |
| ethanol | –114 | 78.3 | 24.5 | 1.66 | 1.35 × 10–9 |
| acetonitrile | –43.8 | 81.6 | 37.5 | 3.45 | 7 × 10–6 |
| dimethyl sulfoxide | 18.5 | 189 | 46.7 | 3.96 | 3 × 10–8 |
| ethylene carbonate | 36.4 | 238 | 89.6 | 4.87 | < 1 × 10–9 |


