17.1: Prelude to Electrochemistry
- Page ID
- 50574
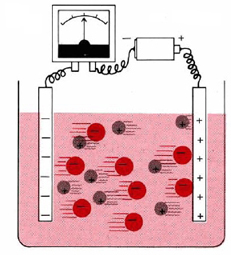
It is also possible to produce a flow of electricity as a result of a spontaneous chemical reaction. A chemical system which can cause a current to flow in this way is called a galvanic cell or a voltaic cell. An example of a galvanic cell with which you are almost certainly familiar is a flashlight battery. Since an electrical current is a flow of electrons or other charged particles, it should come as no surprise that both electrolytic and galvanic cells involve redox reactions.
In an electrolytic cell electric energy supplied from an outside source causes a nonspontaneous reaction to occur. A galvanic (or voltaic) cell, on the other hand. harnesses a spontaneous reaction to produce electric current. In either kind of cell the electrode at which oxidation occurs is called the anode and the electrode at which reduction occurs is the cathode.
Electrolytic cells have numerous commercial applications. Chlorine, sodium hydroxide, hydrogen, aluminum, magnesium, sodium, calcium, and high-purity copper are some of the more important chemicals produced by electrolysis. Electroplating of metals such as chromium, silver, nickel, zinc, and tin is also quite important. In any electrolysis reaction the amount of substance consumed or produced can be related to the electric charge which passes through the cell by means of the Faraday constant F, which equals 9.649 × 104 C mol–1.
A galvanic cell may be represented by an abbreviated notation such as
\[ \text{Zn}│\text{Zn}^{2+} (1\; M)║ \text{Ag}^{2+}(1\; M)│\text{Ag} \label{1} \]
When a cell is written this way, \( \dfrac{1}{2} \)it is always assumed that the left-hand electrode is an anode and an oxidation half-equation occurs there. The right-hand electrode must then be taken as the cathode and a reduction half-equation is assumed to occur there. The cell reaction is the sum of these two half-equations. If it is spontaneous, our assumptions about anode on the left and cathode on the right were correct. Electrons will be forced into an external circuit on the left and the cell emf is taken to be positive. If the cell reaction written according to the above convention turns out to be nonspontaneous, then its reverse will be spontaneous. Our assumptions about which electrode is the anode and which the cathode must also be reversed, and the cell emf is given a negative sign.
Equation \(\ref{1}\) is lots of fun. Because cell emf values indicate whether a process is spontaneous, they are quite useful. They are additive and are conventionally reported as standard electrode potentials. These refer to the emf of a cell with a hydrogen-gas electrode on the left and the electrode whose potential is reported on the right. The standard electrode potential is directly related to the standard free energy change for a reaction, thus allowing direct determination of ΔG°.
Many galvanic cells are of commercial importance. These include dry cells, mercury cells, rechargeable Ni-Cd batteries, and lead storage cells. Fuel cells, in which a continuous supply of both oxidizing and reducing agent is supplied, may eventually become important because of their high efficiencies.


