14.9: Indicators
- Page ID
- 49692
We mentioned that in most titrations it is necessary to add an indicator which produces a sudden color change at the equivalence point. A typical indicator for acid-base titrations is phenolphthalein, HC20H13O4. Phenolphthalein, whose structure is shown below, is a colorless weak acid (Ka = 3 × 10–10 mol/L). Its conjugate base, C20H13O4– has a strong pinkish-red color. In order to simplify, we will write the phenolphthalein molecule as HIn (protonated indicator) and its pink conjugate base as In–. In aqueous solution, phenolphthalein will present the following equilibrium
\[\ce{HIn + H_{2}O \rightleftharpoons In^{-} + H_{3}O^{+}}\label{1} \]
According to Le Chatelier’s principle, the equilibrium expressed in equation \(\ref{1}\) will be shifted to the left if H3O+ is added. Thus in a strongly acidic solution we expect nearly all the pink In– to be consumed, and only colorless HIn will remain. On the other hand, if the solution is made strongly basic, the equilibrium will shift to the right because OH– ions will react with HIn molecules, converting them to In–. Thus the phenolphthalein solution will become pink.
Clearly there must be some intermediate situation where half the phenolphthalein is in the acid form and half in the colored conjugate-base form. That is, at some pH
\[\ce{[HIn] = [In^{-}]} \nonumber \] This intermediate pH can be calculated by applying the Henderson-Hasselbalch equation to the indicator equilibrium:
\[\text{pH}=\text{p}K_{a}\text{ + log}\frac{[\text{ In}^{-}]}{[\text{ HIn }]} \nonumber \]
Thus at the point where half the indicator is conjugate acid and half conjugate base,
\[\text{pH}=\text{p}K_{a}\text{+log1}=\text{p}K_{a} \nonumber \]
For phenolphthalein, we have
\[\text{pH}=\text{p}K_{a}=-\text{log(3 }\times \text{ 10}^{-10}\text{)}=\text{9.5} \nonumber \]
so we expect phenolphthalein to change color in the vicinity of pH = 9.5.
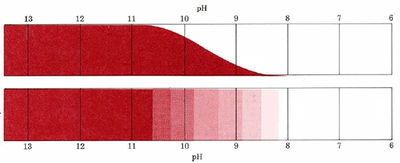
The way in which both the color of phenolphthalein and the fraction present as the conjugate base varies with the pH is shown in detail in Figure \(\PageIndex{1}\). The change of color occurs over quite a limited range of pH―roughly pKa ± 1. In other words the color of phenolphthalein changes perceptibly between about pH 8.3 and 10.5. Observe the actual color change for this indicator in Figure \(\PageIndex{2}\).
Other indicators behave in essentially the same way, but for many of them both the acid and the conjugate base are colored. Their pKa’s also differ from phenolphthalein, as shown in the following table. The indicators listed have been selected so that their pKa values are approximately two units apart. Consequently, they offer a series of color changes spanning the whole pH range.
Properties of Selected Indicators
| Color | ||||
| Name | pKa | Effective pH range | Acid form | Basic form |
| Thymol blue | 1.6 | 1.2 - 2.8 | Red | Yellow |
| Methyl orange | 4.2 | 3.1 - 4.4 | Red | Orange |
| Methyl red | 5.0 | 4.2 - 6.2 | Red | Yellow |
| Bromothymol blue | 7.1 | 6.0 - 7.8 | Yellow | Blue |
| Phenophthalein | 9.5 | 8.3 - 10.0 | Colorless | Red |
| Alizarin yellow | 11.0 | 10.1 - 12.4 | Yellow | Red |
Indicators are often used to make measurements of pH which are precise to about 0.2 or 0.3 units. Suppose, for example, we add two drops of bromothymol blue to a sample of tap water and obtain a green-blue solution. Since bromothymol blue is green at a pH of 6 and blue at a pH of 8, we conclude that the pH is between these two limits. A more precise result could be obtained by comparing the color in the tap water with that obtained when two drops of indicator solution are added to buffer solutions of pH 6.5 and 7.5.
If a careful choice of both colors and pKa is made, it is possible to mix several indicators and obtain a universal indicator which changes color continuously over a very wide pH range. With such a mixture it is possible to find the approximate pH of any solution within this range. So-called pH paper, as seen below, is impregnated with one or several indicators. When a strip of this paper is immersed in a solution, its pH can be judged from the resulting color.
What indicator, from those listed in the table, would you use to determine the approximate pH of the following solutions:
a) 0.1 M CH3COONa (sodium acetate) b) 0.1 M CH3COOH (acetic acid) c) A buffer mixture of sodium acetate and acetic acid d) 0.1 M NH4Cl (ammonium chloride)Solution
a) A solution of sodium acetate will be mildly basic with a pH of 9 or 10. Phenolphthalein would probably be best. b) A solution of acetic acid, unless very dilute, has a pH in the vicinity of 3. Both thymol blue and methyl orange should be tried. c) Since Ka for acetic acid is 1.8 × 10–5 mol/L, we can expect this buffer to have a pH not far from 5. Methyl red would be a good indicator to try. d) Since NH4+is a very weak acid, this solution will be only faintly acidic with a pH of 5 or 6. Again methyl red would be a good indicator to try, though bromothymol blue is also a possibility.

