12.4: Group IIIA
- Page ID
- 49503
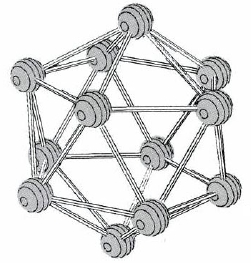
The elements of group IIIA show considerable variability in properties from top to bottom of the periodic table. B is a semimetal or metalloid, and the element has a covalent network structure in which icosahedrons of boron atoms (Figure \(\PageIndex{1}\)) are linked together. None of its compounds contain B3+ ions—covalent bonding is the rule there as well. Many B compounds, especially those containing O, are similar to those of Si (another diagonal relationship) instead of Al. In elemental Al the atoms are closest packed. Al is definitely metallic, but like Be, to which it is related diagonally, it forms many compounds with extensive covalent character.
The other three elements in the group, Ga, In, and Tl, are also metals, but their chemistry is affected to some extent by the fact that they follow the transition elements in the periodic system (Table \(\PageIndex{1}\)). The presence of a filled d subshell, as opposed to only s and p subshells in B and Al, introduces some new atomic properties.
| Element | Symbol | Electron Configuration | Usual Oxidation State | Radius/pm | |
|---|---|---|---|---|---|
| Atomic | Ionic (M3+) | ||||
| Boron | B | [He]2s22p1 | +3 | 80 | - |
| Aluminum | Al | [Ne]3s23p1 | +3 | 125 | 50 |
| Gallium | Ga | [Ar]4s23d104p1 | +3 | 124 | 62 |
| Indium | In | [Kr]5s24d105p1 | +3, +1 | 142 | 81 |
| Thallium | Tl | [Xe]6s24f145d106p1 | +1, +3 | 144 | 95 |
Some properties of the group IIIA elements are shown in Table \(\PageIndex{2}\). In this case the trends are not quite what might have been expected on the basis of previous experience with the periodic table. Both ionization energy and electronegativity decrease significantly from B to Al, but as one proceeds on down the group, these atomic properties change very little. Indeed electronegativity increases from Ga to In to Tl. There is also a break in the expected steady increase in atomic radius down the group. Al and Ga have nearly identical radii, and the ionic radii of A13+ and Ga3+ differ by only 8 pm. In the case of Tl the most common oxidation state is +1,corresponding to loss of only the 6p valence electron, rather than an oxidation state of +3, which would entail loss of the 6s2 pair as well.
| Symbol | Ionization Energy/MJ mol–1 | Density/ g cm–3 | Electro- negativity | Melting Point (in °C) | ||
|---|---|---|---|---|---|---|
| First | Second | Third | ||||
| B | 0.807 | 2.433 | 3.666 | 2.34 | 2.0 | 2300 |
| Al | 0.584 | 1.823 | 2.751 | 2.70 | 1.5 | 660 |
| Ga | 0.585 | 1.985 | 2.969 | 5.90 | 1.6 | 30 |
| In | 0.565 | 1.827 | 2.711 | 7.31 | 1.7 | 157 |
| Tl | 0.596 | 1.977 | 2.884 | 11.83 | 1.8 | 304 |
Many of these anomalies can be understood if we look more closely at the electron configurations of Ga, In, and Tl. In each case a d subshell has been filled between the ns2 pair and the final np1 valence electron. The 4f subshell also intervenes in the building-up process for Tl. Since d and f electrons are not as efficient as s or p electrons at screening nuclear charge, the valence electrons of Ga, In, and Tl are more strongly attracted, the atoms are smaller than would be expected, and ionization energies are unusually large.
Another factor affecting the chemistry of the group IIIA elements is the relative sizes of the first, second, and third ionization energies. These are roughly in the ratio 1:3:4.5 for all elements, and the large increase from first to second ionization energy becomes more pronounced toward the bottom of the group. In the case of Tl, whose large radius prevents close approach and strong bonding to other atoms, the energy required to unpair or ionize the 6s2 pair of electrons and use them to form bonds is often too large to be compensated by the bond energies of the two additional bonds. Consequently Tl+ is the more common oxidation state, rather than Tl3+. This reluctance of the 6s2 pair of electrons to be used in bonding is called the inert-pair effect. It also affects the chemistry of Hg, Pb, and Bi, elements which are adjacent to Tl in the sixth period.
Chemical Reactions and Compounds
Unlike groups IA and IIA, none of the group IIIA elements react directly with hydrogen to form hydrides. The halides of B, Al, and Ga will react with sodium hydride, however, to form tetrahydro anions:
\[\text{4NaH} + \text{BF}_3 \rightarrow \text{NaBH}_4 + \text{3NaF} \nonumber \]
The compounds NaBH4, NaAlH4, and NaGaH4 do not contain H– ions. Instead the hydrogens are covalently bonded to the group IIIA atom:
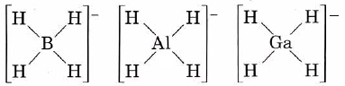
All these anions are tetrahedral, as would be predicted by VSEPR theory.
The tetrahydroaluminate and tetrahydrogallate ions react readily with H2O, splitting the H2O molecule so that the H ends up with another H to form H2, while the OH ends up with the group IIIA atom:
\[\text{AlH}_4^{-}(aq) + \text{4HOH}(l) \rightarrow \text{Al(OH)}_3(s) + \text{OH}^{-}(aq) + \text{4H}_2(g) \nonumber \]
Splitting of an H2O molecule (hydrolysis) by these compounds is similar to the hydrolysis of esters. The hydrolysis of AlH4– is violently explosive, making it necessary to handle NaAlH4 in a dry environment. The anion in NaBH4 by contrast, involves bonds with greater covalent character and is much less readily hydrolyzed.
Only B forms a wide variety of hydride compounds. The simplest of these is diborane, B2H6, a volatile, readily hydrolyzed compound which may decompose explosively. Diborane is made as follows:
\[\ce{3NaBH_{4} + 4BF_{3} \rightarrow 3NaBF_{2} + 2B_{2}H_{6}} \nonumber \]
Diborane is electron deficient—if you try to draw a Lewis diagram, you will soon find it to be impossible—and for a number of years theoretical chemists were mystified about what held the atoms together.
The current picture of bonding in diborane is shown in the Figure \(\PageIndex{2}\) and the accompanying Jmol Applet. Each B is assumed to be surrounded by four sp3 hybrid orbitals. Four of the eight sp3 hybrids from the two B’s overlap the 1s orbitals of four H’s, forming four B—H bonds, all of which lie in a plane. The other four sp3 hybrids overlap between the B’s, forming two banana bonds, a form we described when discussing the double bonds of ethene. Each of the remaining two H nuclei is embedded in the middle of one of the banana bonds. In this way the two electrons in each banana bond hold three nuclei (two B’s and one H) together, and the bond is called a three-center two-electron bond.
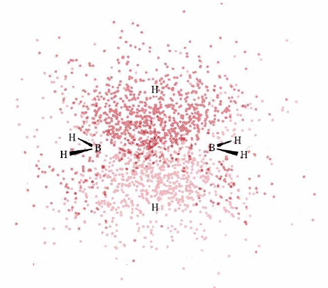
Below is a 3D Jmol model of diborane. We've added a molecular electrostatic potential surface function, as well as molecular orbitals. Let us look at the MEP options first.
- Before looking at any of the surface options, set the minimum cutoff, at the bottom of the MEP options to -0.0100 and the maximum cutoff to 0.0100. Then click "set this cutoff on surface' to apply it. Look at the "MEP on isopotential surface." This will give a good sense of the shape of the potential distribution. You may also want to look at "MEP on Van der Waals Surface". There are two informative "MEP on a plane" surfaces to look at. Click "XY" and "Set Plane Equation" to see the electrostatic potential in the plane of the hydrogen atoms not in the banana bonds. Click "XZ" and "Set Plane Equation" to see the electrostatic potential in the plane containing the banana bond. What do these electrostatic visualizations tell you about diborane?
- While each of the molecular orbitals is informative, for our purposes, look at N5. What does the shape of this orbital mean for the nature of the banana bond?
Solution
a) All three show that the boron atoms are surrounded by negative charge, the free hydrogen atoms are neutral, and the two hydrogen atoms in the banana bonds are electron deficient. This picture makes sense in terms of our discussion on diborane structure. First, the two banana bond hydrogen atoms are sharing an electron pair not just with one boron atom, but with two. As you can see in the dot density diagram, the electron density for this bond is pulled away from the hydrogen atoms. The negative charge on boron arises from their being four, instead of three, bonds to the atom.
b)This orbital spans the region between the hydrogen atom and both borons. Since orbitals contain only 2 electrons, this means that there is a strong bonding presence of only two electrons shared between 3 atoms (rather than 2 individual bonds of 2 electrons each).
All the group IIIA elements form trihalides, although Tl does so reluctantly, preferring to remain in the +1 oxidation state. The general reaction is
\[\text{2M}(s) + \text{3X}_2 \rightarrow \text{2MX}_3 \nonumber \] M = B, Al, Ga, In, Tl X = F, Cl, Br, I
Below is a video example of one such reaction, that of Al and Br2:
In this video, aluminum foil is cut into smaller squares, and placed in a watch glass with liquid bromine, also giving off gaseous bromine vapor fumes. Initially, no reaction occurs, and the aluminum foil has a protective oxide coating. Soon, white fumes start to come off, signaling the start of the reaction. Since the reaction is exothermic, more bromine fumes also emerge. The aluminum and bromine react violently, causing flashes of light. After the reaction has occurred, a fan is used to remove the fumes, so that the AlBr3 product may be seen. The overall reaction is:
\[\text{2Al}(s) + \text{3Br}_2 \rightarrow \text{2AlBr}_3 \nonumber \]
All the boron halides consist of discrete BX3 molecules, which are electron deficient. Below is a 3D model of one of these halides,BCl3. As predicted by VSEPR theory the molecule has a trigonal planar structure. On this model, Molecular Electrostatic Potential Surface options have been provided. First, set the minimum cutoff to -0.0100 and the maximum cutoff to 0.0100. Again, any of the three surface potential options are useful, but in this case, "MEP on Isopotential Surface" is most informative. First, all three chlorides have a partial negative charge. The boron halides are strong Lewis acids because each can accept an electron pair to complete an octet on B. Looking at the positive isopotential surface around the boron atom, it is clear how open each side of the molecule is to accepting an electron pair.
The bromides and iodides of Al, Ga, In, and Tl are primarily covalent, and two MX3 molecules usually combine to form a dimer (dimer means two units):

In the dimer structure a lone electron pair from a Br atom in one AlBr3 unit has been donated to the Al of the other AlBr3 unit, and vice versa. This forms two coordinate covalent bonds to hold the dimer together. (These bonds are shown as arrows.)
The fluorides of Al, Ga, In, and Tl are primarily ionic. Only the larger, more easily polarizable Br– and I– ions can be distorted enough by the small M3+ ions to give mainly covalent bonds. Most of the chlorides are border-line cases. This is reflected in the melting points:
AlF3, 1291°C AlCl3, 190°C Al2Br6, 97.5°C Al2I6, 191°C
Solid AlCl3 consists of A13+ and Cl– ions, but in the liquid and gas phases Al2Br6 dimers predominate.
The oxides and oxyanions of B and Al are the main compounds of commercial importance. Borax, Na2B4O7•10H2O, is the principal ore of B, and Al is obtained from bauxite, Al2O3•xH2O. Bauxite is an example of a hydrous oxide. It contains an indeterminate amount of water (xH2O) in the crystal lattice. Although Al is the most abundant metal in the earth’s crust, most of it is found in complicated aluminosilicates (feldspars). It is extremely difficult to convert the feldspars to Al metal, and any Si which remains as an impurity greatly degrades the strength and other properties of Al. Consequently the metal is obtained from bauxite, of which there is only a limited supply.
The first step in the recovery of Al is called the Bayer process. This depends on the fact that Al2O3 is amphoteric; that is, it can behave as either an acid or a base. If it behaves as a base, it can react with, and dissolve in, acidic solutions. If it behaves as an acid, it can dissolve in basic solutions. The amphoteric behavior of Al2O3 comes about because of the presence of A13+ ions and O2– ions. Al3+ has a very small radius and a large ionic charge, and so it strongly attracts H2O molecules—so strongly that the Al(H2O)63+ ion can donate protons. (Other cations which do this were described in Sec. 11.3.) Oxide ion, of course, is a good proton acceptor and a strong base.
The Bayer process makes use of the acidic behavior of Al2O3 by dissolving it in strong base:
\[\text{Al}_2\text{O}_3(s) + \text{3H}_2\text{O}(l) + \text{2OH}^{-}(aq) \rightarrow \text{2Al(OH)}_4^{-}(aq) \nonumber \]
The oxides of most metals are strongly basic and do not dissolve, while the oxides of nonmetals such as Si are acidic and more soluble in base than Al2O3. Thus a fairly pure sample of aluminum hydroxide, Al(OH)3, can be obtained by filtering off the basic oxides and acidifying the solution slightly. CO2, an acidic oxide, is used for this purpose:
\[\text{CO}_2(g) + \text{Al(OH)}_4^{-}(aq) \rightarrow \text{Al(OH)}_3(s) + \text{HCO}_3^{-}(aq) \nonumber \]
The Al(OH)3 is then heated to drive off H2O:
\[\text{2Al(OH)}_3(s) \xrightarrow{\Delta } \text{Al}_2\text{O}_3(s) + \text{3H}_2{O}(g) \nonumber \]
And Al is obtained by the Hall-Heroult process:
\[\text{Al}_2\text{O}_3(l) \xrightarrow{\text{electrolysis}} \text{2Al}(l) + \text{3H}_2\text{O}(g) \nonumber \]
This process is described in greater detail in the section on aluminum production.
The last step in aluminum production requires tremendous quantities of electrical energy. This, as well as the scarcity of its ore, makes aluminum more expensive than iron, the only metal which is more widely used. Nevertheless, aluminum has several advantages over iron. One is its considerably lower density, making possible alloys of comparable strength with considerably lower mass. A second advantage of aluminum is the coating of Al2O3 which forms on its surface when it is exposed to air. This coating sticks to the surface and is insoluble in neutral solutions, and so it prevents further oxidation. Iron, by contrast, forms a hydrous oxide (rust) which flakes off easily, exposing additional metal to the air. Thus under normal environmental conditions aluminum will last much longer than iron. This is a problem when aluminum beverage cans litter roadsides, but in general it makes aluminum a very favorable candidate for recycling.


