10.12: Boiling Point
- Page ID
- 49666
When we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. However, the pressure inside the bubbles must also be equal to the external pressure above the liquid. If this were not so, the bubbles would either suddenly collapse or suddenly expand. It follows therefore that when a liquid boils, the vapor pressure of the liquid is equal to the external pressure.
Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kPa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point which is usually quoted in chemical literature. Not everyone lives at sea level, though. Denver, Colorado, for example, is about a mile high, and the average atmospheric pressure there is only 630 mmHg (84 kPa). Liquids attain a vapor pressure of 630 mmHg at a somewhat lower temperature than is required to produce 760 mmHg (1 atm). Consequently liquids in Denver boil some 4 to 5°C lower than the normal boiling point. Since the boiling point is often used to identify a liquid, chemists living at high altitudes must be careful to allow for this difference.
The dependence of the boiling point on the external pressure can often be very useful. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. Such a liquid can often be distilled at reduced pressure. The temperature of boiling is then much lower, and the risk of decomposition considerably less. The reverse procedure is used in a pressure cooker. The pressure inside the sealed cooker builds up until it is larger than atmospheric, and so the water used for cooking boils at a temperature above its normal boiling point. Therefore the cooking proceeds more rapidly.
The following video highlights the idea that boiling point is dependent upon both temperature and pressure. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. When cold water is poured over the top of the flask, it cools the gas above the liquid water. This decreases the vapor pressure above the water. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur.
Video \(\PageIndex{1}\): So you thought you could only boil water with heat? Watch as we flip that upside down and use ice cubes. Keep in mind there is a little Science involved and all the items you need are already in your home. We take the glass bottle fill it 1/3 with water set on your stove top until a nice boil 100 ºC remove with oven mit let cool for 5-10 seconds then put cap back on top. Invert bottle into a cold bowl of ice with ice cubes on the bottom and watch as the water starts to re boil in front of your eyes.
From the figure displaying boiling points of four alkanes, estimate the boiling points of the four alkanes when the pressure is reduced to 600 mmHg.
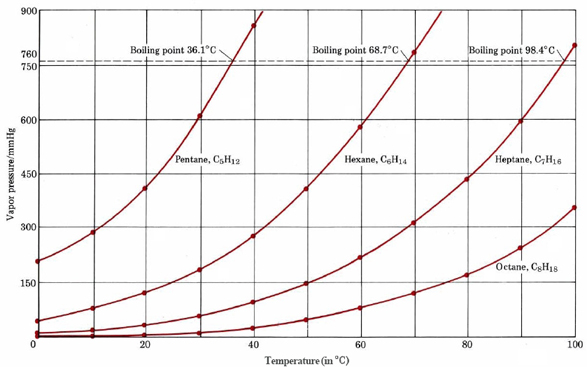
Solution
Reading along the 600-mmHg line in the graph, we find that it meets the vapor-pressure curve for pentane at about 29°C. Accordingly this is the boiling point of pentane at 600 mmHg. Similarly we find the boiling point of hexane to be 61°C, and of heptane to be 90°C. The boiling point of octane is above 100°C and cannot be estimated from the graph.


