8.11: Hydrogen Bonding- Water
- Page ID
- 49448
It would seem that the London forces and dipole forces discussed in the earlier sections of this chapter should be adequate to account for macroscopic properties of covalently bonded substances. Certainly they can be successfully applied to hydrocarbons and many polar substances. There are some experimental data, however, which cannot be explained by London and dipole forces alone. An example appears below, where boiling points are plotted for hydrogen compounds (hydrides) of most of the nonmetals.
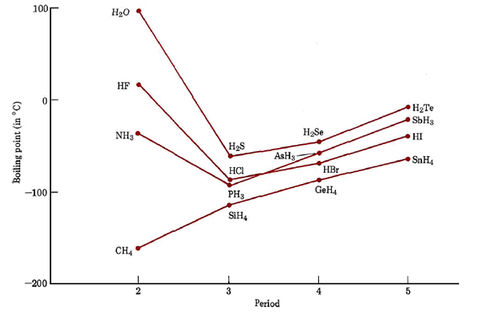
Hydrides of elements in the fifth period behave as we might predict. SnH4, which consists of nonpolar molecules, boils at the lowest temperature. SbH3, H2Te and HI, all of which are polar, have somewhat higher boiling points, but all lie within a range of 50°C. Similar behavior occurs among the hydrides of elements in the fourth and third periods. In the second period, however, the polar hydrides NH3, H2O, and HF all have boiling points more than 100°C above that of the nonpolar compound CH4. Clearly these second-row hydrides must have particularly strong intermolecular forces.
In order to see why this happens, let us consider the simplest second-row hydride—HF. Suppose that two HF molecules approach each other, as shown in the following figure. In each HF molecule, the hydrogen nucleus is rather poorly shielded by a thin electron cloud (only two electrons), and much of that electron cloud has been distorted toward the highly electronegative fluorine atom.
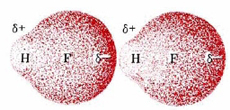
Consequently, the hydrogen nucleus in the HF on the right can approach much closer to the fluorine side of the left-hand molecule than could any other kind of nucleus. Also, because fluorine is such an electronegative atom, its partial negative charge is particularly large. The attractive force between the electrons of the left-hand fluorine and the right-hand hydrogen nucleus will be unusually strong, and there will be considerable distortion of the electron clouds. Some covalent-bond character develops between fluorine in one molecule and hydrogen in the other.
The close approach of oppositely charged ends of molecular dipoles combines with this small degree of covalent-bond character to produce an abnormally strong intermolecular force called a hydrogen bond. In order for hydrogen bonding to occur, there must be a hydrogen atom connected to a small, highly electronegative atom (usually fluorine, oxygen, or nitrogen) in one molecule. The other molecule must have a very electronegative atom (again usually fluorine, oxygen, or nitrogen) which has one or more lone pairs of electrons. Separation of two molecules joined by a hydrogen bond requires 10 to 30 kJ mol–1, roughly 10 times the energy needed to overcome dipole forces. Thus hydrogen bonding can account for the unusually high boiling points of NH3, H2O, and HF.
Hydrogen bonding between HF molecules is particularly evident in solid HF, where the atoms are arranged in a zigzag pattern:
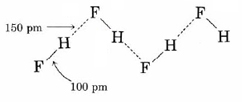
Here the distance between hydrogen and fluorine nuclei in different molecules is only 150 pm. If we add the van der Waals radii for hydrogen and fluorine, we obtain an expected hydrogen to fluorine distance of (120 + 135) pm = 255 pm, over 100 pm larger than that observed. Obviously the hydrogen and fluorine atoms in adjacent molecules are not just “touching,” but must be associated in a much more intimate way.
Provisional Redefinition of the Hydrogen Bond
A task force of the International Union of Pure and Applied Chemistry (IUPAC) is proposing a redefinition of the hydrogen bond:
"The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X-H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation." [1]
This proposed redefinition would include many more situations where hydrogen bonding appears to be important, including cases where the hydrogen atom is attracted to atoms other than F,O, and N. For example, there appears to be an attraction between the d subshell electrons in the central platinum atom in the complex shown in the Figure below, and the hydrogen atoms of an adjacent H2O or the NH3 group on an adjacent platinum complex[2].
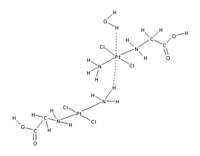
The new formulation recognizes that the hydrogen bond may have some covalent character[3][4]. It recognizes that hydrogen bonds may be significant in H2S at higher pressures and low temperatures, or that a "dihydrogen bond" may form (where metal hydrides like LiH are the H bond acceptors).
References
- media.iupac.org/reports/provisional/abstract11/arunan_310311.html
- Kozelka, J. et al, Angew. Chem. Int. Ed., DOI:10.1002/anie.201001892
- Hydrogen Bond [en.Wikipedia.org]
- Kemsley, Jyllian, "Hydrogen Bond Formation", Chemical & Engineering News, November 22, 2010, p. 32


