6.2: Ionic Bonding
- Page ID
- 49341
Ionic bonding involves transfer of an electron from one atom (which becomes a positively charged cation) to another (which becomes a negatively charged anion). The two ions attract strongly to form a crystal lattice.
Since ionic bonding requires that the atoms involved have unequal attraction for their valence electrons, an ionic compound must involve atoms of two quite different elements. Attraction for electrons depends on the distance of the electrons from the nucleus (which in turn depends on the amount of shielding by inner electrons). Ionic compounds generally form between metals toward the left and bottom of the periodic table, and nonmetals toward the right and top of the periodic table.
The simplest example of a binary ionic compound is provided by the combination of elements number 1 (H) and number 3 (Li) in lithium hydride, LiH. On a microscopic level the formula LiH contains four electrons. In separate Li and H atoms these electrons are arranged as shown in part a of the following figure. The H atom has the electron configuration 1s1, and Li is 1s22s1. When the two atoms are brought close enough together, however, the striking rearrangement of the electron clouds shown part b takes place. Here the color coding shows clearly that the electron density which was associated with the 2s orbital in the individual Li atom has been transferred to a 1s orbital surrounding the H atom. As a result, two new microscopic species are formed. The extra electron transforms the H atom into a negative ion or anion, written H– and called the hydride ion. The two electrons left on the Li atom are not enough to balance the charge of +3 on the Li nucleus, and so removal of an electron produces a positive ion or cation, written Li+ and called the lithium ion. The electron-transfer process can be summarized in of Lewis diagrams as follows:
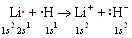
The opposite charges of Li+ and H–attract each other strongly, and the ions form an ion pair (see image below) in which the two nuclei are separated by a distance of 160 pm (1.60 Å).
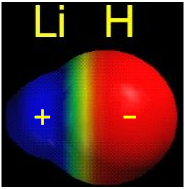
The image above shows an ion pair of Lithium Hydride. Notice how Lithium has a strong positive charge (cation) and Hydrogen has a strong negative charge (anion).
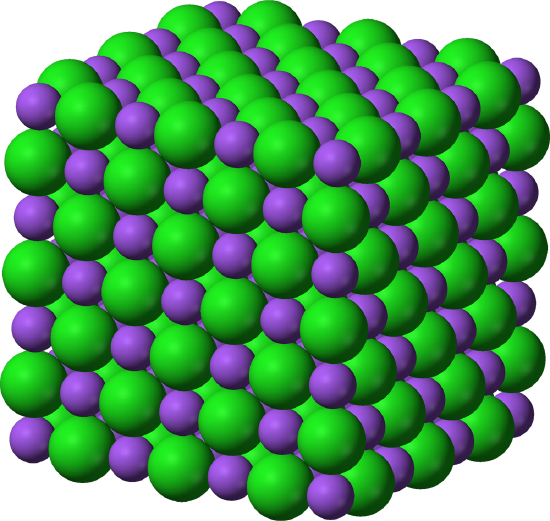
Multiple ion pairs (as seen in the image above) connect to form a crystal lattice, pictured below. All ionic solids form a crystal lattice and the shape of the lattice determines the properties and look of the solid.