Characteristic Reactions of Tin Ions (Sn²⁺, Sn⁴⁺)
- Page ID
- 97278
- Most common oxidation states: +2, +4
- M.P. 232º
- B.P. 2270º
- Density 7.30 g/cm3
- Characteristics: Metallic tin is soft and malleable. It slowly dissolves in dilute nonoxidizing acids or more readily in hot concentrated \(\ce{HCl}\). It reacts with \(\ce{HNO3}\) to form metastannic acid, \(\ce{H2SnO3}\), a white substance insoluble in alkalies or acids. In neutral or only slightly acidic solutions, zinc displaces tin from its compounds, forming the metal.
Characteristic reactions of Sn²⁺ and Sn⁴⁺
In aqueous solutions, both tin(II) and tin(IV) exist as complex ions. Both tin(II) chloride and tin(IV) chloride tend to undergo hydrolyze and aged solutions of these salts become measurably acidic. Acid should be added to aqueous solutions of these compounds to prevent hydrolysis.
Tin(IV) chloride exists as a colorless liquid. It is soluble in organic solvents, and is a nonconductor of electricity, indicating that it is a molecular compound. Tin(II) chloride is a strong reducing agent and is easily oxidized by atmospheric oxygen. Metallic tin is often added to solutions of \(\ce{SnCl2}\) to prevent this oxidation.
Chloride Ion
Although there is no visible reaction, tin(II) exists as the complex ion \(\ce{[SnCl4]^{2-}}\) and tin(IV) as the complex ion \(\ce{[SnCl6]^{2-}}\).
Aqueous Ammonia
Aqueous ammonia precipitates white \(\ce{Sn(OH)2}\) and white \(\ce{Sn(OH)4}\) with tin(II) and tin(IV), respectively.
\[\ce{[SnCl4]^{2-}(aq) + 2NH3(aq) + 2H2O(l) <=> Sn(OH)2(s) + 2NH4^{+}(aq) + 4Cl^{-}(aq)} \nonumber \]
\[\ce{[SnCl6]^{2-}(aq) + 4NH3(aq) + 4H2O(l) <=> Sn(OH)4(s) + 4NH4^{+}(aq) + 6Cl^{-}(aq)} \nonumber \]
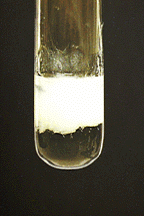
Both precipitates, tin(II) hydroxide and tin(IV) hydroxide, dissolve in excess aqueous ammonia.
Sodium Hydroxide
Sodium hydroxide also precipitates the hydroxides:
\[\ce{[SnCl4]^{2-}(aq) + 2OH^{-}(aq) <=> Sn(OH)2(s) + 4Cl^{-}(aq)} \nonumber \]
\[\ce{[SnCl6]^{2-}(aq) + 4OH^{-}(aq) <=> Sn(OH)4(s) + 6Cl^{-}(aq)} \nonumber \]

These precipitates dissolve in excess hydroxide:
\[\ce{Sn(OH)2(s) + 2OH^{-}(aq) <=> [Sn(OH)4]^{2-}(aq)} \nonumber \]
\[\ce{Sn(OH)4(s) + 2OH^{-}(aq) <=> [Sn(OH)6]^{2-}(aq) } \nonumber \]

Hydrogen Sulfide
In mildly acidic solution, sulfide precipitates \(\ce{SnS}\) (brown) and \(\ce{SnS2}\) (light yellow):
\[\ce{[SnCl4]^{2-}(aq) + H2S(aq) <=> SnS(s) + 2H^{+}(aq) + 4Cl^{-}(aq)} \nonumber \]

\[\ce{[SnCl6]^{2-}(aq) + 2H2S(aq) <=> SnS2(s) + 4H^{+}(aq) + 6Cl^{-}(aq)} \nonumber \]

\(\ce{SnS2}\) is soluble in basic solutions containing excess \(\ce{S2^{-}}\), even in the presence of ammonia. It is also soluble in 6 M \(\ce{HCl}\):
\[\ce{SnS2(s) + S2^{-}(aq) <=> [SnS3]^{2-}(aq)} \nonumber \]
\[\ce{SnS2(s) + 4H^{+}(aq) + 6Cl^{-}(aq) <=> [SnCl6]^{2-}(aq) + 2H2S(aq)} \nonumber \]

\(\ce{SnS}\) is soluble in 12 M \(\ce{HCl}\):
\[\ce{SnS(s) + 2H^{+}(aq) + 4Cl^{-}(aq) <=> [SnCl4]^{2-}(aq) + H2S(aq)} \nonumber \]

Reducing and Oxidizing Agents
In \(\ce{HCl}\) solution, either metallic \(\ce{Fe}\) or metallic \(\ce{Al}\) will reduce \(\ce{Sn(IV)}\) to \(\ce{Sn(II)}\):
\[\ce{Fe(s) + [SnCl6]^{2-}(aq) -> Fe^{2+}(aq) + [SnCl4]^{2-}(aq) + 2Cl^{-}(aq)} \nonumber \]

\[\ce{2Al(s) + 3[SnCl6]^{2-}(aq) --> 2Al^{3+}(aq) + 3[SnCl4]^{2-}(aq) + 6Cl^{-}(aq)} \nonumber \]


Sn(II) reduces \(\ce{HgCl2}\) to \9\ce{Hg2Cl2}\) (white) or metallic mercury (black) or a mixture of both. These reactions are described in more detail in the mercury section.

In basic solution, \(\ce{Sn(II)}\) reduces \(\ce{Bi(III)}\) to metallic \(\ce{Bi}\). This reaction is described in more detail in the bismuth section.

No Reaction
\(\ce{Cl^{-}}\), \(\ce{SO4^{2-}}\)


